Abstract
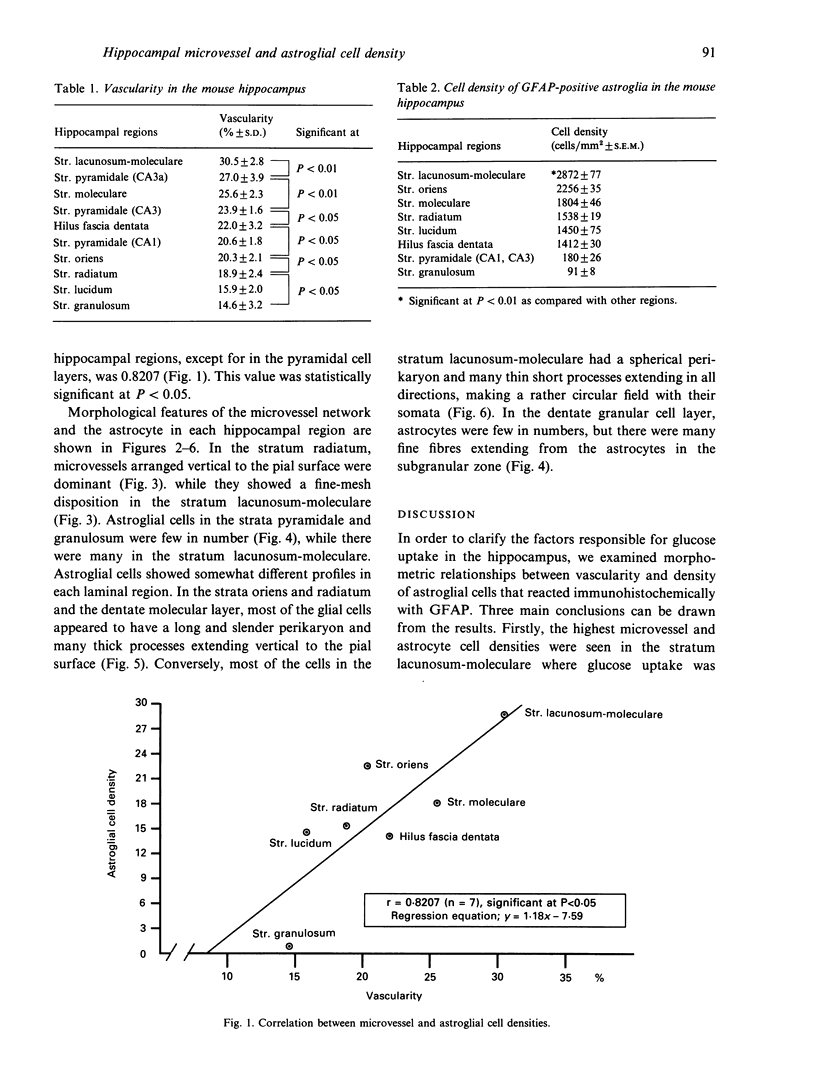
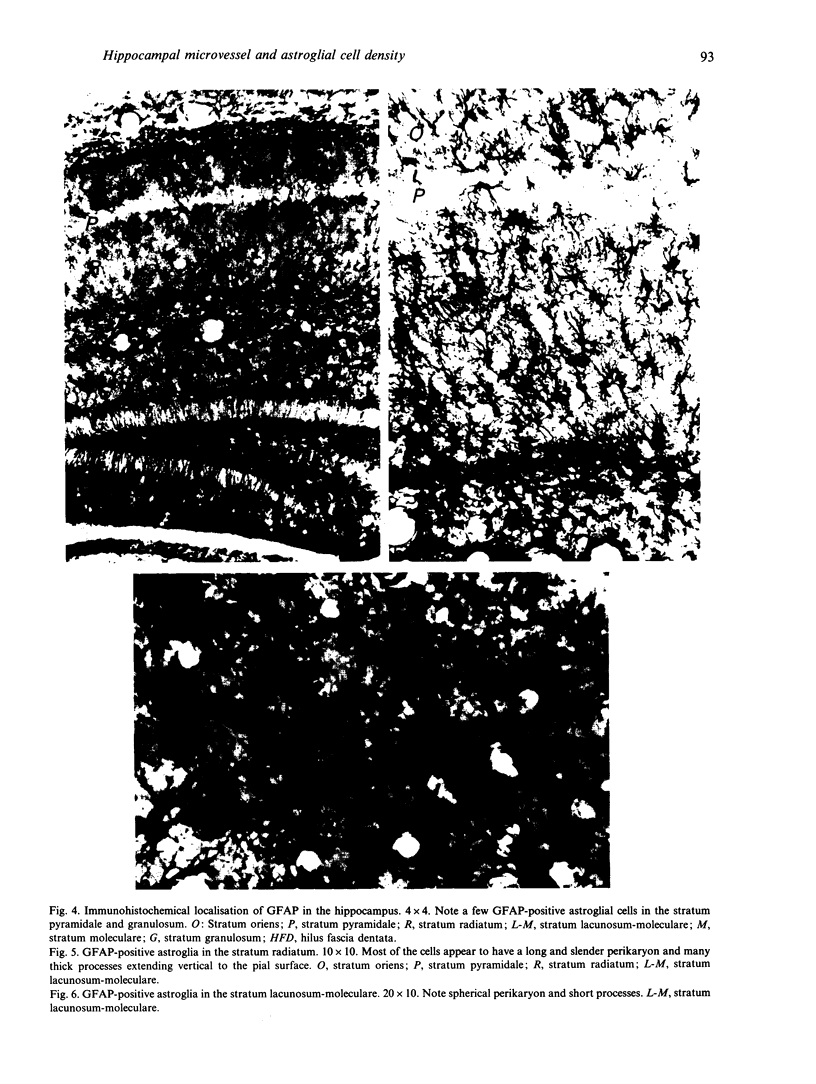
In order to study the factors responsible for glucose uptake in the mouse hippocampus, microvessel and astroglial cell densities were measured and compared in each laminal region. Microvessel density was examined on histologically prepared sections after injection of Indian ink and measured by means of an image analyser. Astroglial cell density was determined after the cells were stained immunohistochemically. Microvessel and astroglial cell densities were determined in 10 different hippocampal structures. Microvessel and astroglial cell densities were strongly correlated in all layers except the pyramidal cell layers. The highest density of perfused microvessels was found in the stratum lacunosum-moleculare, compared with other regions, and the lowest values were found in the stratum lucidum and dentate granular cell layer. Among pyramidal cell layers, microvessel density in sector CA3a was significantly higher than that in CA1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Holmqvist B., Voorhoeve P. E. Excitatory synapses on hippocampal apical dendrites activated by entorhinal stimulation. Acta Physiol Scand. 1966 Apr;66(4):461–472. doi: 10.1111/j.1748-1716.1966.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Borowsky I. W., Collins R. C. Histochemical changes in enzymes of energy metabolism in the dentate gyrus accompany deafferentation and synaptic reorganization. Neuroscience. 1989;33(2):253–262. doi: 10.1016/0306-4522(89)90204-2. [DOI] [PubMed] [Google Scholar]
- Borowsky I. W., Collins R. C. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989 Oct 15;288(3):401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Cordero M. E., Zvaighaft A., Muzzo S., Brunser O. Histological maturation of astroglial cells in the archicortex of early malnourished rats. Pediatr Res. 1982 Mar;16(3):187–191. doi: 10.1203/00006450-198203000-00005. [DOI] [PubMed] [Google Scholar]
- DeLeo J., Toth L., Schubert P., Rudolphi K., Kreutzberg G. W. Ischemia-induced neuronal cell death, calcium accumulation, and glial response in the hippocampus of the Mongolian gerbil and protection by propentofylline (HWA 285). J Cereb Blood Flow Metab. 1987 Dec;7(6):745–751. doi: 10.1038/jcbfm.1987.129. [DOI] [PubMed] [Google Scholar]
- Eckenhoff M. F., Rakic P. Radial organization of the hippocampal dentate gyrus: a Golgi, ultrastructural, and immunocytochemical analysis in the developing rhesus monkey. J Comp Neurol. 1984 Feb 10;223(1):1–21. doi: 10.1002/cne.902230102. [DOI] [PubMed] [Google Scholar]
- FRIEDE R. L., FLEMING L. M., KNOLLER M. A comparative mapping of enzymes involved in hexosemonophosphate shunt and citric acid cycle in the brain. J Neurochem. 1963 Apr;10:263–277. doi: 10.1111/j.1471-4159.1963.tb05042.x. [DOI] [PubMed] [Google Scholar]
- Gross P. M., Sposito N. M., Pettersen S. E., Panton D. G., Fenstermacher J. D. Topography of capillary density, glucose metabolism, and microvascular function within the rat inferior colliculus. J Cereb Blood Flow Metab. 1987 Apr;7(2):154–160. doi: 10.1038/jcbfm.1987.38. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- Kageyama G. H., Wong-Riley M. T. Histochemical localization of cytochrome oxidase in the hippocampus: correlation with specific neuronal types and afferent pathways. Neuroscience. 1982 Oct;7(10):2337–2361. doi: 10.1016/0306-4522(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Klein B., Kuschinsky W., Schröck H., Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol. 1986 Dec;251(6 Pt 2):H1333–H1340. doi: 10.1152/ajpheart.1986.251.6.H1333. [DOI] [PubMed] [Google Scholar]
- Kosaka T., Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986 Jul 8;249(2):242–260. doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979 Apr;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Ribak C. E. The histochemical localization of cytochrome oxidase in the dentate gyrus of the rat hippocampus. Brain Res. 1981 May 11;212(1):169–174. doi: 10.1016/0006-8993(81)90046-9. [DOI] [PubMed] [Google Scholar]
- Shimada M., Shimono R., Ozaki H. S. Freeze-mount microautoradiographic study in the mouse hippocampus after intravenous injection of tritiated 2-deoxyglucose and glucose. Neuroscience. 1989;31(2):347–354. doi: 10.1016/0306-4522(89)90378-3. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Griffioen A. W., Jorritsma-Byham B., Krijnen J. L. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci Lett. 1988 Feb 29;85(2):193–198. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Groenewegen H. J. Laminar origin and septotemporal distribution of entorhinal and perirhinal projections to the hippocampus in the cat. J Comp Neurol. 1984 Apr 10;224(3):371–385. doi: 10.1002/cne.902240305. [DOI] [PubMed] [Google Scholar]








