Abstract
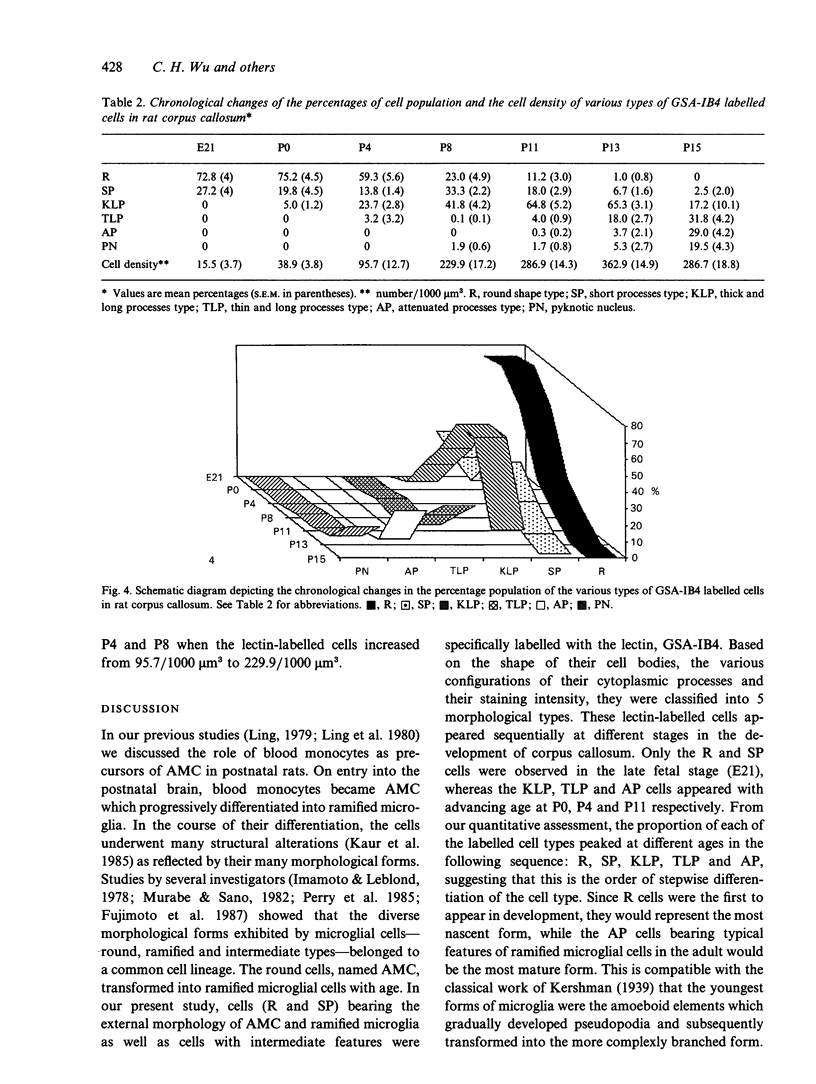
The morphometric and quantitative changes associated with the differentiation of amoeboid microglia into ramified microglial cells in the corpus callosum of rats between 21 d postconception (E21) and 15 d postnatally are described. Using lectin labelling, 5 morphological types of labelled cells (R, SP, KLP, TLP, AP) based on cell body shape, the configuration of their cytoplasmic processes and their staining intensity, were recognised. Round cells (R) and cells with stout processes (SP) were aggregated in the central part of the developing corpus callosum whereas the highly branched labelled cells were distributed at its periphery. When the morphometric data and labelling intensities of labelled cells were analysed with the aid of an image analysis system, the values for cell length, area and perimeter increased as the complexity of branching increased, whereas the lectin-labelling intensity became reduced. Quantitative study showed that the proportion of the different morphological types of lectin-labelled cells peaked at different ages. The sequential peaking of R, SP and highly branched cells with advancing age suggests a similar chronological order of differentiation of R into branched cells. The quantitative study also showed a rapid increase in the density of lectin-labelled cells in the postnatal period between P4 and P8, attributed primarily to the active proliferation of the cell type. The consequent reduction of cell density (after P13) was probably due to cell death, a feature which appeared to increase with development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell K. W., Holländer H., Streit W., Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2(5):437–448. doi: 10.1017/s0952523800012335. [DOI] [PubMed] [Google Scholar]
- Ashwell K. Development of microglia in the albino rabbit retina. J Comp Neurol. 1989 Sep 15;287(3):286–301. doi: 10.1002/cne.902870303. [DOI] [PubMed] [Google Scholar]
- Boya J., Calvo J. L., Carbonell A. L., Borregon A. A lectin histochemistry study on the development of rat microglial cells. J Anat. 1991 Apr;175:229–236. [PMC free article] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39(2):451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Fujimoto E., Miki A., Mizoguti H. Histochemical studies of the differentiation of microglial cells in the cerebral hemispheres of chick embryos and chicks. Histochemistry. 1987;87(3):209–216. doi: 10.1007/BF00492411. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Lawson L. J., Perry V. H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Brain macrophages in rats following intravenous labelling of mononuclear leucocytes with colloidal carbon. J Anat. 1978 Jan;125(Pt 1):101–106. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Penney D., Leblond C. P. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the 'ameboid cells' present in the corpus callosum of postnatal rats. J Comp Neurol. 1980 Oct 1;193(3):631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Transformation of monocytes into amoeboid microglia in the corpus callosum of postnatal rats, as shown by labelling monocytes by carbon particles. J Anat. 1979 Jun;128(Pt 4):847–858. [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Tsuchihashi Y., Kitamura T., Fujita S. Immunohistochemical studies of blood monocytes infiltrating into the neonatal rat brain. Acta Neuropathol. 1984;62(4):291–297. doi: 10.1007/BF00687611. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. VI. Postnatal development of microglial cells. Cell Tissue Res. 1982;225(3):469–485. doi: 10.1007/BF00214798. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Hume D. A., Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985 Jun;15(2):313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Light microscopic identification of immature glial cells in semithin sections of the developing mouse corpus callosum. J Anat. 1976 Dec;122(Pt 3):521–537. [PMC free article] [PubMed] [Google Scholar]




