Abstract
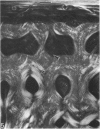
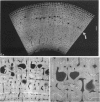
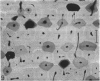
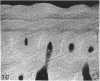
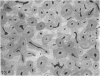
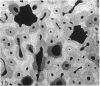
The dorsal cortex of the equine third metacarpal mid-diaphyseal bone was characterised during growth by the histological and microradiographic examination of specimens from 30 horses ranging in age from 2 months to 8 y. Bone from horses aged less than 6 months was characterised by rapid periosteal apposition of circumferential trabeculae of woven bone that were next connected by radial trabeculae to the parent cortex. Deposition of lamellar bone on the inner trabecular surfaces resulted in rows of primary osteons. Replacement of primary bone occurred only after 4 months of age and preferentially in the woven interstitial bone separating rows of primary osteons formed in the postnatal periosteal cortex. Resorption cavities and incompletely filled secondary osteons characterised bone of 1 and 2-y-old horses. Bone from horses older than 3 y contained several generations of secondary osteons, fewer resorption spaces and incompletely filled osteons, and had a greater portion of circumferentially oriented collagen fibres than bone from younger horses. Bone from horses older than 5 y had large resorption cavities characterised by irregular boundaries. We propose that the process of periosteal bone tissue apposition observed in growing foals be called 'saltatory primary osteonal bone formation' and that this process results in faster cortical expansion and larger total surface area for bone deposition than circumferential lamellar, simple primary osteonal, and plexiform mechanisms of periosteal bone formation. We speculate that bone from 1 and 2-y-old horses would be more susceptible to fatigue microdamage resulting from compressive loads because of high porosity, few completed secondary osteons and low proportion of circumferentially oriented collagen fibres.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arsenault A. L. Vascular canals in bovine cortical bone studied by corrosion casting. Calcif Tissue Int. 1990 Nov;47(5):320–325. doi: 10.1007/BF02555916. [DOI] [PubMed] [Google Scholar]
- Burr D. B., Martin R. B., Schaffler M. B., Radin E. L. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18(3):189–200. doi: 10.1016/0021-9290(85)90204-0. [DOI] [PubMed] [Google Scholar]
- CURREY J. D. Differences in the tensile strength of bone of different histological types. J Anat. 1959 Jan;93(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Carter D. R., Hayes W. C. Compact bone fatigue damage: a microscopic examination. Clin Orthop Relat Res. 1977;(127):265–274. [PubMed] [Google Scholar]
- Carter D. R., Hayes W. C. Fatigue life of compact bone--I. Effects of stress amplitude, temperature and density. J Biomech. 1976;9(1):27–34. doi: 10.1016/0021-9290(76)90136-6. [DOI] [PubMed] [Google Scholar]
- Carter D. R., Spengler D. M. Mechanical properties and composition of cortical bone. Clin Orthop Relat Res. 1978 Sep;(135):192–217. [PubMed] [Google Scholar]
- ENLOW D. H. A study of the post-natal growth and remodeling of bone. Am J Anat. 1962 Mar;110:79–101. doi: 10.1002/aja.1001100202. [DOI] [PubMed] [Google Scholar]
- ENLOW D. H. Functions of the Haversian system. Am J Anat. 1962 May;110:269–305. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- EVANS F. G. Relations between the microscopic structure and tensile strength of human bone. Acta Anat (Basel) 1958;35(4):285–301. doi: 10.1159/000141417. [DOI] [PubMed] [Google Scholar]
- Evans F. G., Vincentelli R. Relations of the compressive properties of human cortical bone to histological structure and calcification. J Biomech. 1974 Jan;7(1):1–10. doi: 10.1016/0021-9290(74)90064-5. [DOI] [PubMed] [Google Scholar]
- FROST H. M. In vivo osteocyte death. J Bone Joint Surg Am. 1960 Jan;42-A:138–143. [PubMed] [Google Scholar]
- Lipson S. F., Katz J. L. The relationship between elastic properties and microstructure of bovine cortical bone. J Biomech. 1984;17(4):231–240. doi: 10.1016/0021-9290(84)90134-9. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Burr D. B. A hypothetical mechanism for the stimulation of osteonal remodelling by fatigue damage. J Biomech. 1982;15(3):137–139. doi: 10.1016/s0021-9290(82)80001-8. [DOI] [PubMed] [Google Scholar]
- Nunamaker D. M., Butterweck D. M., Provost M. T. Fatigue fractures in thoroughbred racehorses: relationships with age, peak bone strain, and training. J Orthop Res. 1990 Jul;8(4):604–611. doi: 10.1002/jor.1100080417. [DOI] [PubMed] [Google Scholar]
- Portigliatti Barbos M., Bianco P., Ascenzi A., Boyde A. Collagen orientation in compact bone: II. Distribution of lamellae in the whole of the human femoral shaft with reference to its mechanical properties. Metab Bone Dis Relat Res. 1984;5(6):309–315. doi: 10.1016/0221-8747(84)90018-3. [DOI] [PubMed] [Google Scholar]
- SMITH J. W., WALMSLEY R. Factors affecting the elasticity of bone. J Anat. 1959 Oct;93:503–523. [PMC free article] [PubMed] [Google Scholar]
- Smith J. W. Collagen fibre patterns in mammalian bone. J Anat. 1960 Jul;94(Pt 3):329–344. [PMC free article] [PubMed] [Google Scholar]
- Turner A. S., Mills E. J., Gabel A. A. In vivo measurement of bone strain in the horse. Am J Vet Res. 1975 Nov;36(11):1573–1579. [PubMed] [Google Scholar]