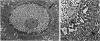Abstract
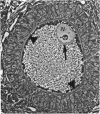
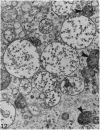
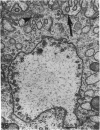
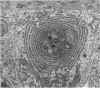
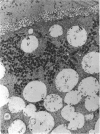
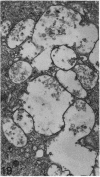
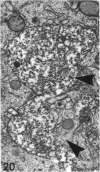
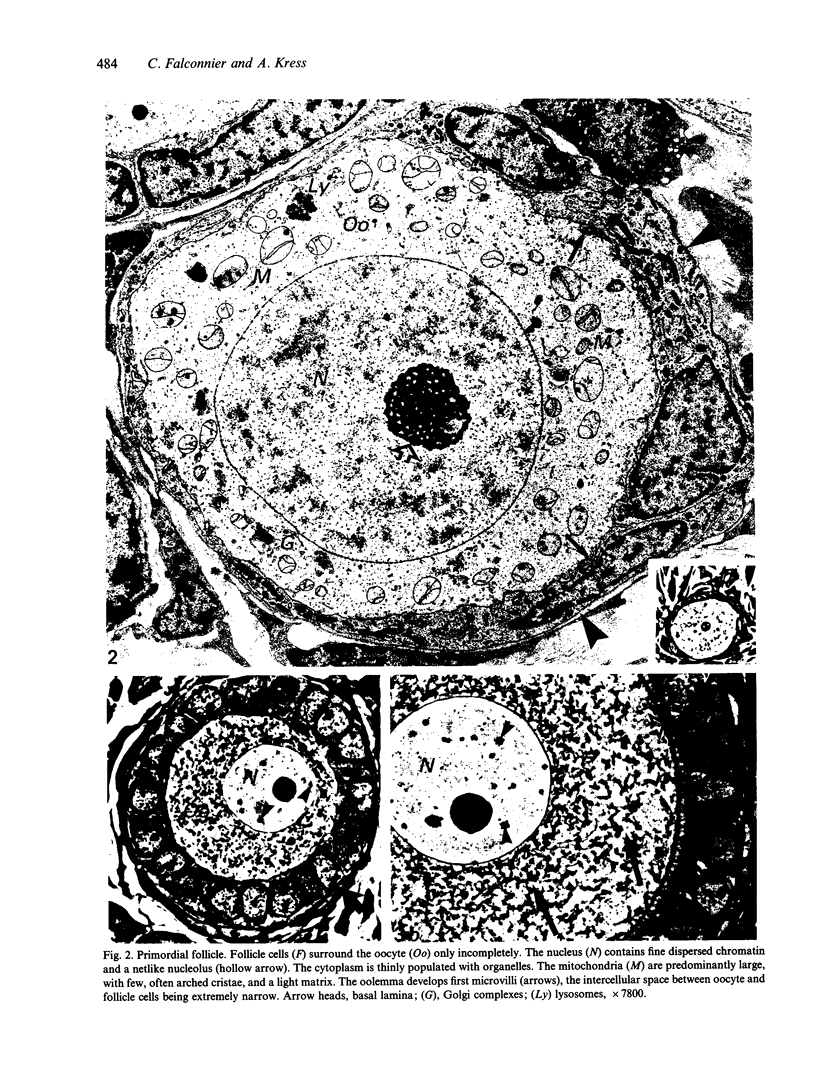
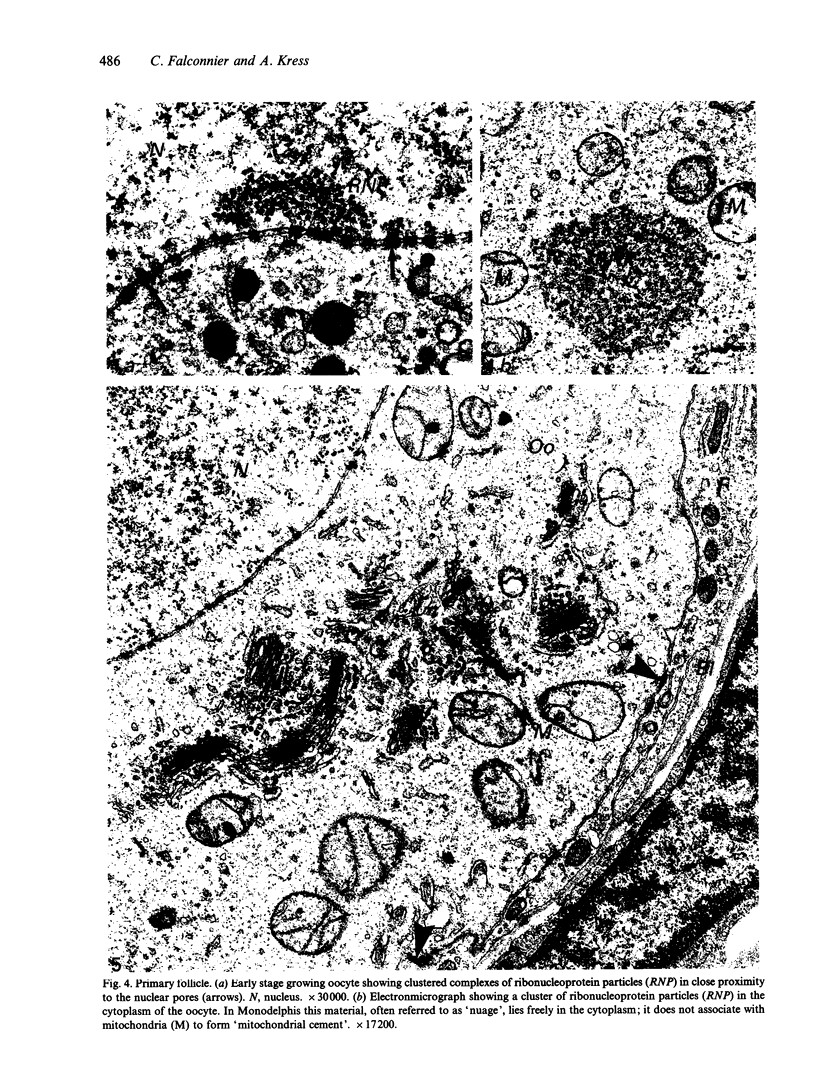
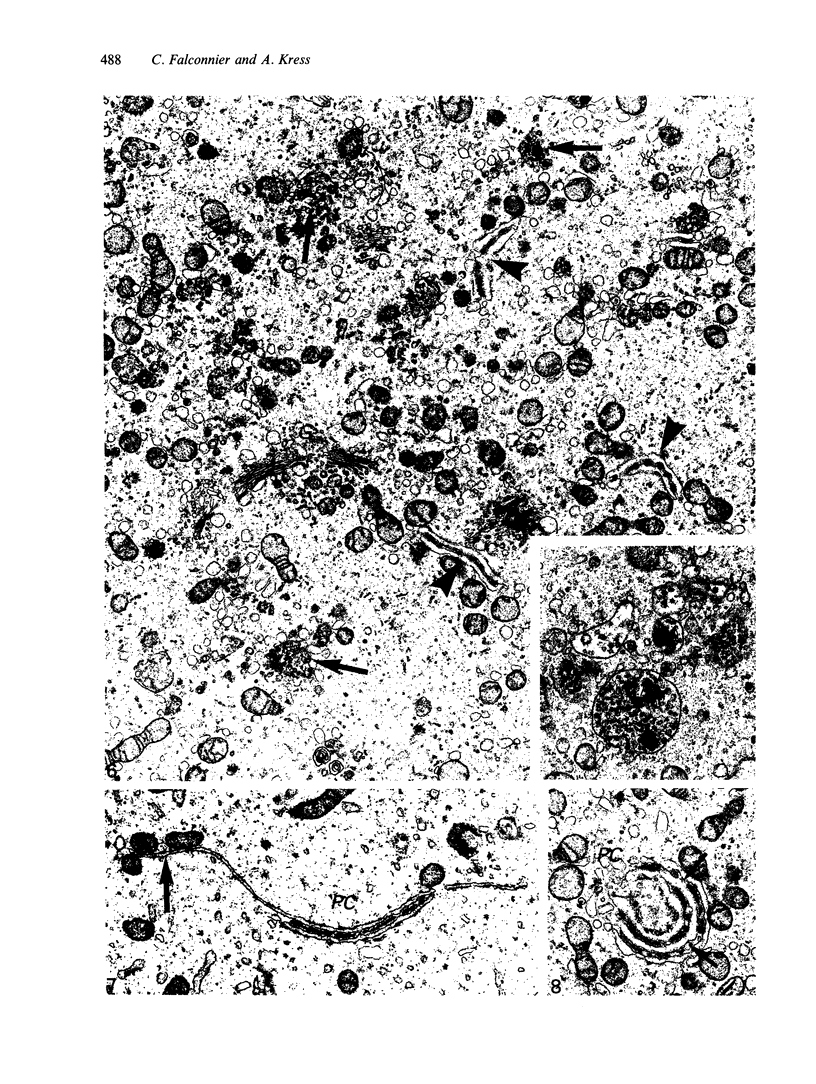
The growth of the opossum Monodelphis oocyte does not correspond to the strict biphasic pattern so far described in eutherians and marsupials. The oocyte increases appreciably in size during the last stage of antral follicle development. During the primordial and primary follicle stage Balbiani bodies or paranuclear complexes are not detectable in Monodelphis oocytes. Organelles are randomly distributed. In addition to the nucleolus, perichromatin and ribonucleoprotein particles are other intranuclear structures which occur as regular components in the early Monodelphis oocyte. Clusters of particles are mostly seen in close association with the nuclear envelope. Similar material has been encountered in the cytoplasm as a type of freely-existing 'nuage' material but never as mitochondria-associated 'nuage' or 'cement'. Both types of particles, intranuclear and cytoplasmic, disappear by the time antral follicle formation begins. Mitochondria are at first of the typical transformed shape seen in most mammalian oocytes. They are large, round or oval in outline with a few, often arched, cristae and a light matrix. During the primary follicle stage, mitochondrial size and complexity decrease and the matrix becomes electron dense. A close relationship between mitochondria and endoplasmic reticulum appears early in the primordial and later in the primary follicle oocyte. Regularly detected structures in the ooplasm of preantrum oocytes are paired or 'confronting' cisternae of endoplasmic reticulum, which are lamellar complexes comprised of 2 or more parallel cisternae with intervening electron-dense material. The most conspicuous inclusions in the Monodelphis oocytes of the tertiary and graafian follicles are vesicles. All other organelles are confined to the peripheral zone of the oocyte. Golgi and endoplasmic vesicles both take part in the formation of multivesicular bodies which seems to be the starting point for the vesicle accumulation. Further increase in size involves the incorporation of endocytotic vesicles and the coalescence of larger vesicles. Ordinary fixation procedure leave the vesicles empty. Cortical granules are found only in small numbers.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. C., HERTIG A. T. STUDIES ON GUINEA PIG OOCYTES. I. ELECTRON MICROSCOPIC OBSERVATIONS ON THE DEVELOPMENT OF CYTOPLASMIC ORGANELLES IN OOCYTES OF PRIMORDIAL AND PRIMARY FOLLICLES. J Cell Biol. 1964 Jun;21:397–427. doi: 10.1083/jcb.21.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate L. A., Stuart T. D., Ley R. D. Ultraviolet radiation-induced histopathological changes in the skin of the marsupial Monodelphis domestica. I. The effects of acute and chronic exposures and of photoreactivation treatment. Br J Dermatol. 1985 Aug;113(2):219–227. doi: 10.1111/j.1365-2133.1985.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Baggott L. M., Davis-Butler S., Moore H. D. Characterization of oestrus and timed collection of oocytes in the grey short-tailed opossum, Monodelphis domestica. J Reprod Fertil. 1987 Jan;79(1):105–114. doi: 10.1530/jrf.0.0790105. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Moore H. D., Penfold L. M., Burgess A. M., Mittwoch U. Gonadal sex differentiation in the neonatal marsupial, Monodelphis domestica. Development. 1990 Jul;109(3):699–703. doi: 10.1242/dev.109.3.699. [DOI] [PubMed] [Google Scholar]
- Breed W. G., Leigh C. M., Bennett J. H. Sperm morphology and storage in the female reproductive tract of the fat-tailed dunnart, Sminthopsis crassicaudata (Marsupialia: Dasyuridae). Gamete Res. 1989 May;23(1):61–75. doi: 10.1002/mrd.1120230107. [DOI] [PubMed] [Google Scholar]
- Breed W. G., Leigh C. M. Morphological changes in the oocyte and its surrounding vestments during in vivo fertilization in the dasyurid marsupial Sminthopsis crassicaudata. J Morphol. 1990 May;204(2):177–196. doi: 10.1002/jmor.1052040207. [DOI] [PubMed] [Google Scholar]
- Burkholder G. D., Comings D. E., Okada T. A. A storage form of ribosomes in mouse oocytes. Exp Cell Res. 1971 Dec;69(2):361–371. doi: 10.1016/0014-4827(71)90236-9. [DOI] [PubMed] [Google Scholar]
- Cran D. G., Moor R. M., Hay M. F. Fine structure of the sheep oocyte during antral follicle development. J Reprod Fertil. 1980 May;59(1):125–132. doi: 10.1530/jrf.0.0590125. [DOI] [PubMed] [Google Scholar]
- Eddy E. M. Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat Rec. 1974 Apr;178(4):731–757. doi: 10.1002/ar.1091780406. [DOI] [PubMed] [Google Scholar]
- Fadem B. H. Evidence for the activation of female reproduction by males in a marsupial, the gray short-tailed opossum (Monodelphis domestica). Biol Reprod. 1985 Aug;33(1):112–116. doi: 10.1095/biolreprod33.1.112. [DOI] [PubMed] [Google Scholar]
- Fadem B. H., Hill H. Z. The gray opossum (Monodelphis domestica): a marsupial model for xenogeneic neoplasms. Cancer Lett. 1985 Jun;27(2):233–238. doi: 10.1016/0304-3835(85)90113-2. [DOI] [PubMed] [Google Scholar]
- Fadem B. H., Rayve R. S. Characteristics of the oestrous cycle and influence of social factors in grey short-tailed opossums (Monodelphis domestica). J Reprod Fertil. 1985 Mar;73(2):337–342. doi: 10.1530/jrf.0.0730337. [DOI] [PubMed] [Google Scholar]
- Fadem B. H., Tesoriero J. V., Whang M. Early differentiation of the gonads in the gray short-tailed opossum (Monodelphis domestica). Biol Neonate. 1992;61(2):131–136. doi: 10.1159/000243542. [DOI] [PubMed] [Google Scholar]
- Fleming M. W., Harder J. D. Luteal and follicular populations in the ovary of the opossum (Didelphis virginiana) after ovulation. J Reprod Fertil. 1983 Jan;67(1):29–34. doi: 10.1530/jrf.0.0670029. [DOI] [PubMed] [Google Scholar]
- Fleming W. N., Saacke R. G. Fine structure of the bovine oocyte from the mature graafian follicle. J Reprod Fertil. 1972 May;29(2):203–213. doi: 10.1530/jrf.0.0290203. [DOI] [PubMed] [Google Scholar]
- Garde M. L. The Ovary of Ornithorhynchus, with special reference to Follicular Atresia. J Anat. 1930 Jul;64(Pt 4):422–453. [PMC free article] [PubMed] [Google Scholar]
- Green D. P. An analysis of the structure of the bilaminar lamellae of the hamster egg. J Ultrastruct Res. 1985 Apr;91(1):30–37. doi: 10.1016/0889-1605(85)90073-4. [DOI] [PubMed] [Google Scholar]
- Guraya S. S. Histochemical study of developing ovarian oocyte of the American opossum. Acta Embryol Morphol Exp. 1968 Jul;10(2):181–191. [PubMed] [Google Scholar]
- Homa S. T., Racowsky C., McGaughey R. W. Lipid analysis of immature pig oocytes. J Reprod Fertil. 1986 Jul;77(2):425–434. doi: 10.1530/jrf.0.0770425. [DOI] [PubMed] [Google Scholar]
- Hubbard G. B., Saphire D. G., Hackleman S. M., Silva M. V., Vandeberg J. L., Stone W. H. Ontogeny of the thymus gland of a marsupial (Monodelphis domestica). Lab Anim Sci. 1991 Jun;41(3):227–232. [PubMed] [Google Scholar]
- Hughes R. L., Shorey C. D. Observations on the permeability properties of the egg membranes of the marsupial, Trichosurus vulpecula. J Reprod Fertil. 1973 Jan;32(1):25–32. doi: 10.1530/jrf.0.0320025. [DOI] [PubMed] [Google Scholar]
- Jones T. E., Munger B. L. Early differentiation of the afferent nervous system in glabrous snout skin of the opossum (Monodelphis domesticus). Somatosens Res. 1985;3(2):169–184. doi: 10.3109/07367228509144582. [DOI] [PubMed] [Google Scholar]
- Kang Y. H., Anderson W. A. Ultrastructure of the oocytes of the Egyptian sping mouse (Acomys cahirinus). Anat Rec. 1975 Jun;182(2):175–200. doi: 10.1002/ar.1091820205. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Cutts J. H. Ultrastructural observations on the shell membrane of the North American opossum (Didelphis virginiana). Anat Rec. 1983 Oct;207(2):335–338. doi: 10.1002/ar.1092070212. [DOI] [PubMed] [Google Scholar]
- Kress A. Ultrastructural studies on oogenesis in the shrew (Crocidura russula): I. The preantral follicle. J Morphol. 1984 Jan;179(1):59–71. doi: 10.1002/jmor.1051790107. [DOI] [PubMed] [Google Scholar]
- Kress A. Ultrastructural study on oogenesis in shrews (Neomys fodiens, Sorex araneus). Acta Anat (Basel) 1984;118(1):42–49. doi: 10.1159/000145820. [DOI] [PubMed] [Google Scholar]
- Ley R. D., Applegate L. A. Ultraviolet radiation-induced histopathologic changes in the skin of the marsupial Monodelphis domestica. II. Quantitative studies of the photoreactivation of induced hyperplasia and sunburn cell formation. J Invest Dermatol. 1985 Oct;85(4):365–367. doi: 10.1111/1523-1747.ep12276992. [DOI] [PubMed] [Google Scholar]
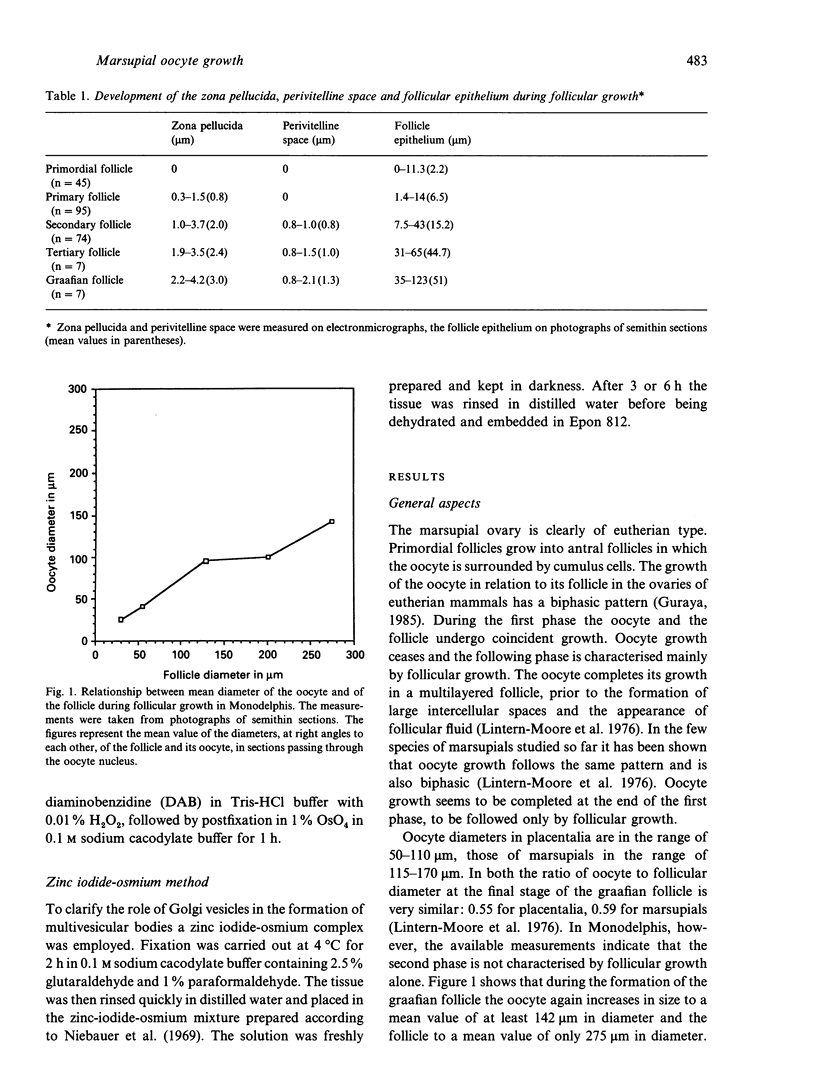
- Lintern-Moore S., Moore G. P., Tyndale-Biscoe C. H., Poole W. E. The growth of the oocyte and follicle in the ovaries of monotremes and marsupials. Anat Rec. 1976 Jul;185(3):325–332. doi: 10.1002/ar.1091850306. [DOI] [PubMed] [Google Scholar]
- Lyne A. G., Hollis D. E. Observations on Graafian follicles and their oocytes during lactation and after the removal of pouch young in the marsupials Isoodon macrourus and Perameles nasuta. Am J Anat. 1983 Jan;166(1):41–61. doi: 10.1002/aja.1001660104. [DOI] [PubMed] [Google Scholar]
- Niebauer G., Krawczyk W. S., Kidd R. L., Wilgram G. F. Osmium zinc iodide reactive sites in the epidermal Langerhans cell. J Cell Biol. 1969 Oct;43(1):80–89. doi: 10.1083/jcb.43.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa T., Sung W. K., Jagiello G. M., Bowne W. A quantitative analysis of mitochondria during fetal mouse oogenesis. J Morphol. 1988 Feb;195(2):225–234. doi: 10.1002/jmor.1051950208. [DOI] [PubMed] [Google Scholar]
- Norberg H. S. The follicular oocyte and its granulosa cells in domestic pig. Z Zellforsch Mikrosk Anat. 1972;131(4):497–517. doi: 10.1007/BF00306967. [DOI] [PubMed] [Google Scholar]
- Norberg H. S. The morphological relationship between mitochondria and cytoplasmic membranes of the follicular oocyte in domestic pig. Z Zellforsch Mikrosk Anat. 1972;124(4):520–531. doi: 10.1007/BF00335255. [DOI] [PubMed] [Google Scholar]
- Olson G. E., Winfrey V. P. Changes in actin distribution during sperm development in the opossum, Monodelphis domestica. Anat Rec. 1991 Jun;230(2):209–217. doi: 10.1002/ar.1092300208. [DOI] [PubMed] [Google Scholar]
- Palombi F., Viron A. Nuclear cytochemistry of mouse oogenesis. 1. Changes in extranucleolar ribonucleoprotein components through meiotic prophase. J Ultrastruct Res. 1977 Oct;61(1):10–20. doi: 10.1016/s0022-5320(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Fadem B. H. The oocyte of a new world marsupial, Monodelphis domestica: structure, formation, and function of the enveloping mucoid layer. J Exp Zool. 1987 Jun;242(3):363–371. doi: 10.1002/jez.1402420316. [DOI] [PubMed] [Google Scholar]
- Samples N. K., Vandeberg J. L., Stone W. H. Passively acquired immunity in the newborn of a marsupial (Monodelphis domestica). Am J Reprod Immunol Microbiol. 1986 Jul;11(3):94–97. doi: 10.1111/j.1600-0897.1986.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Stern S., Biggers J. D., Anderson E. Mitochondria and early development of the mouse. J Exp Zool. 1971 Feb;176(2):179–191. doi: 10.1002/jez.1401760206. [DOI] [PubMed] [Google Scholar]
- Takeuchi I. K., Sonta S., Takeuchi Y. K. Association of perichromatin granules with nuclear pores of growing Chinese hamster oocytes. J Electron Microsc (Tokyo) 1984;33(4):388–394. [PubMed] [Google Scholar]
- Talbot P., DiCarlantonio G. Ultrastructure of opossum oocyte investing coats and their sensitivity to trypsin and hyaluronidase. Dev Biol. 1984 May;103(1):159–167. doi: 10.1016/0012-1606(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Tesoriero J. V. Early ultrastructural changes of developing oocytes in the dog. J Morphol. 1981 May;168(2):171–179. doi: 10.1002/jmor.1051680206. [DOI] [PubMed] [Google Scholar]
- Ullmann S. L. Observations on the primordial germ cells of bandicoots (Peramelidae, Marsupialia). J Anat. 1981 Jun;132(Pt 4):581–595. [PMC free article] [PubMed] [Google Scholar]
- Ullmann S. L. Observations on the primordial oocyte of the bandicoot Isoodon macrourus (Peramelidae, Marsupialia). J Anat. 1979 May;128(Pt 3):619–631. [PMC free article] [PubMed] [Google Scholar]
- Ullmann S. L. Ovary development in bandicoots: sexual differentiation to follicle formation. J Anat. 1989 Aug;165:45–60. [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M., Josefowicz W. J. Oocyte development in the mouse: an ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978 May;156(2):209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Weakley B. S. Basic protein and ribonucleic acid in the cytoplasm of the ovarian oocyte in the golden hamster. Z Zellforsch Mikrosk Anat. 1971;112(1):69–84. doi: 10.1007/BF00665622. [DOI] [PubMed] [Google Scholar]
- Weakley B. S. Electron microscopy of the oocyte and granulosa cells in the developing ovarian follicles of the golden hamster (Mesocricetus auratus). J Anat. 1966 Jul;100(Pt 3):503–534. [PMC free article] [PubMed] [Google Scholar]
- Weakley B. S. Granular cytoplasmic bodies in oocytes of the golden hamsters during the post-natal period. Z Zellforsch Mikrosk Anat. 1969;101(3):394–400. doi: 10.1007/BF00335575. [DOI] [PubMed] [Google Scholar]
- Weakley B. S., James J. L., Webb P. Paired cisternae of endoplasmic reticulum in developing ovarian oocytes of the golden hamster. Acta Anat (Basel) 1986;125(4):258–262. doi: 10.1159/000146173. [DOI] [PubMed] [Google Scholar]
- Weakley B. S. Variations in mitochondrial size and ultrastructure during germ cell development. Cell Tissue Res. 1976 Jul 6;169(4):531–550. doi: 10.1007/BF00218151. [DOI] [PubMed] [Google Scholar]
- Wischnitzer S. Intramitochondrial transformations during oocyte maturation in the mouse. J Morphol. 1967 Jan;121(1):29–46. doi: 10.1002/jmor.1051210104. [DOI] [PubMed] [Google Scholar]
- de Loos F., van Vliet C., van Maurik P., Kruip T. A. Morphology of immature bovine oocytes. Gamete Res. 1989 Oct;24(2):197–204. doi: 10.1002/mrd.1120240207. [DOI] [PubMed] [Google Scholar]