Abstract
Central nervous system development requires precise and localized regulation of neural precursor behaviour. Here we show how the interaction between growth factor and integrin signalling pathways provides a mechanism for such precision in oligodendrocyte progenitor (OP) proliferation. While physiological concentrations of platelet-derived growth factor (PDGF) were not in themselves sufficient to promote OP proliferation, they did so on extracellular matrix (ECM) substrates that bind αvβ3 integrin. Upon PDGF-AA exposure and αvβ3 engagement, a physical co-association between both receptors was demonstrated, confirming the interaction between these signalling pathways. Furthermore, we found that PDGFαR stimulated a protein kinase C-dependent activation of integrin αvβ3, which in turn induced OP proliferation via a phosphatidylinositol 3-kinase-dependent signalling pathway. These studies establish a mechanism by which OP proliferation is dependent on the availability of both an ECM ligand and a mitogenic growth factor. Growth factor- mediated integrin activation is the critical integrative step in proliferation signalling, and ensures that the response of neural precursor cells to long-range cues can be regulated by their cellular neighbours, allowing precise control of cell behaviour during development.
Keywords: activation/extracellular matrix/integrin/PDGF/WOW-1
Introduction
Growth factors play essential roles in the control of cell behaviour during neural development. During myelination, for example, they have been implicated in the regulation of oligodendrocyte precursor (OP) proliferation, migration, survival and differentiation (Armstrong et al., 1990; Barres and Raff, 1994; Bansal and Pfeiffer, 1997; Osterhout et al., 1997; Butt and Berry, 2000; Garcion et al., 2001). However, signals deriving from diffusible molecules such as growth factors may act over significant distances, and the precise control over individual cell behaviour required for correct development will require additional, more localized mechanisms. One potential means of providing this localization is the extracellular matrix (ECM), cues from which have been shown to contribute to the regulation of many developmental processes (Adams and Watt, 1993; DeSimone, 1994; Hynes, 1994). Integration of short-range cues from the ECM with the longer range growth factor signals provides a mechanism by which the cells can communicate with, and respond to, both adjacent and more distant cellular neighbours. The identification and characterization of any such integrative pathways is therefore essential for understanding the regulation of development in the central nervous system (CNS) and other systems.
To define these mechanisms of integration, we have examined the regulation of OP proliferation. As well as providing an essential mechanism to increase appropriately the number of precursor cells that can differentiate into myelin-forming oligodendrocytes (Barres and Raff, 1994), the regulation of OP proliferation also defines an important switching point in the oligodendroglial lineage. Proliferating OPs do not differentiate and still have the potential to revert to a stem cell phenotype (neural precursor cell) (Kondo and Raff, 2000). In contrast, OPs that have ceased to divide constitutively differentiate into a myelin-forming oligodendrocyte unless inhibitory cues such as Notch signalling pathways are present (Temple and Raff, 1985; Wang et al., 1998). Both localized ECM cues and soluble growth factor signals have been implicated in the regulation of proliferation. OPs in cell culture proliferate in response to a number of different growth factors, including platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs), insulin-like growth factors (IGFs) and neuregulin (NRG) (McMorris and Dubois-Dalcq, 1988; Richardson et al., 1988; Bogler et al., 1990; Canoll et al., 1996). In the case of PDGF, experiments with transgenic mice lacking PDGF-A have shown that PDGF is crucial for OP proliferation in vivo (Fruttiger et al., 1999). Evidence for a role for the ECM comes from studies on mice deficient in tenascin-C, which show reduced levels of OP proliferation in vivo (Garcion et al., 2001). At least in cell culture, this effect is mediated by the αvβ3 integrin, which we have also identified previously as a regulator of OP proliferation using an overexpression strategy (Blaschuk et al., 2000). One mechanism for the integration of short- and long-range signalling in OPs is therefore via integrin–growth factor interactions, specifically between the PDGFαR [which is the only PDGF receptor (PDGFR) expressed on OPs; Pringle et al., 1989] and αvβ3 integrin. Here we provide experimental support for this mechanism by demonstrating a functional and physical link between the two receptors in OPs. The biological significance of this interaction is demonstrated by our findings that physiological concentrations of PDGF do not trigger proliferation directly, but do so indirectly via activation of αvβ3 integrin that leads to increased affinity for ligand. As a result, the mitogenic response to PDGF at these physiological concentrations is absolutely dependent on the availability of an appropriate integrin ligand. This interaction ensures that the immediate cellular environment regulates growth factor-stimulated proliferation, and the central role of integrins in this regulation also provides a mechanism for the further integration of other signalling cues, the different downstream pathways of which can lead to changes in integrin activation.
Results
Vitronectin potentiates oligodendrocyte progenitor proliferation at physiological PDGF concentrations
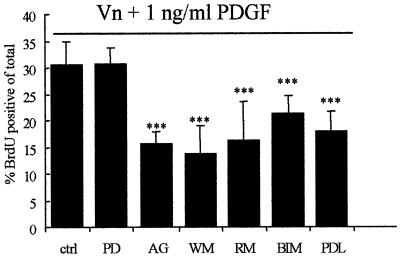
To examine the effects of physiological concentrations of PDGF on OP proliferation, it is necessary to estimate the concentration present in vivo during development. Recent results examining cell cycle times in OPs show that these are regulated by the availability of PDGF, and the cell cycle times in vivo suggest that the concentration of PDGF available to OPs is <1 ng/ml (van Heyningen et al., 2001). To examine cell proliferation at these physiological concentrations, we initially used primary OP cells obtained from cultures of cortical cells by mechanical dissociation and not subjected to any prior growth factor expansion (McCarthy and de Vellis, 1980; Milner and ffrench-Constant, 1994). Such expansion may alter the expression levels of growth factor receptors, making the primary cells a better model of the situation in vivo. In experiments using minimal medium [Dulbecco’s modified Eagle’s medium (DMEM) supplemented only with glutamine and antibiotics, and therefore lacking any serum and other potential signalling factors], we found that concentrations of ≤1 ng/ml PDGF did not promote proliferation on non-specific poly-d-lysine (PDL) substrates (Figure 1). As our previous studies implicate the αvβ3 integrin in OP proliferation (Blaschuk et al., 2000; Garcion et al., 2001), we next investigated whether proliferation in response to these concentrations of PDGF could be observed when signalling from αvβ3 integrin was initiated by ligation to a vitronectin (Vn) substrate. As shown in Figure 1, OPs were unable to proliferate on Vn in the absence of growth factors, whereas they readily attached to the substrate. However, with the addition of physiological concentrations of PDGF (≤1 ng/ml) to these adherent OPs, proliferation was now observed. In contrast, no proliferation was seen if the cells were exposed to the growth factor prior to plating on the Vn substrate. This substrate-dependent proliferation response was due to a specific cross-talk between PDGF and Vn-induced signalling as other described OP or stem cell mitogens, FGF-2, epidermal growth factor (EGF) and NRG (Bogler et al., 1990; Reynolds and Weiss, 1992; Canoll et al., 1996; Tropepe et al., 1999), were not able to induce proliferation on Vn at these growth factor concentrations. Similar results to those with Vn were observed on fibronectin (Fn) (not shown) but, as shown in Figure 1, ≤1 ng/ml PDGF did not induce OP proliferation on PDL or laminin-1 (Ln-1). OP proliferation does occur in DMEM on PDL or Ln-1 at higher, non-physiological, PDGF levels (10 ng/ml) (data not shown, but see Baron et al., 2000). This concentration of PDGF is widely used to grow OPs in cell culture, and we conclude that proliferation observed under these conditions may be regulated by signalling pathways that differ from those activated by physiological growth factor concentrations.
Fig. 1. Effect of different combinations of substrates and growth factors (at suboptimal concentrations) on OP proliferation. OPs were plated on different ECM substrates (Vn and Ln-1) or PDL, after which they were exposed to growth factors (PDGF-AA, FGF-2, NRG and EGF). The effect of these substrate–growth factor combinations on OP proliferation was determined after 16 h by BrdU incorporation, as described in Materials and methods. Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (***P <0.001) for the indicated growth factor concentration and substrate as compared with the PDL control at the same growth factor concentration. Note that the proliferation was significantly increased on a Vn substrate in the presence of PDGF-AA.
Integrin αvβ3 mediates the PDGF-induced oligodendrocyte progenitor proliferation on Vn
The requirement for an ECM substrate, Vn, for OP proliferation at ≤1 ng/ml PDGF suggests that αv integrins, all of which will bind Vn, are involved in the potentiation of the growth factor response. In confirmation of this, we found that RGD peptides that will competitively inhibit Vn binding to all αv integrins (Ruoslahti, 1996) inhibited this proliferation (Figure 2). Oligodendroglial cells express four αv integrins, αvβ1, αvβ3, αvβ5 and αvβ8 (Milner and ffrench-Constant, 1994; Milner et al., 1997). To investigate which of these Vn receptors was responsible, bromodeoxyuridine (BrdU) incorporation assays were performed in the presence of specific blocking anti-integrin monoclonal antibodies against αvβ1, αvβ3 and αvβ5 (blocking antibodies against αvβ8 are not yet available). As shown in Figure 2A, an antibody against a functional epitope on integrin αvβ3 (F11) inhibited the PDGF-induced proliferation. This antibody had no effect on the PDGF-induced proliferation at higher PDGF levels on a PDL or Ln-1 substrate (data not shown), as expected, since these substrates are not ligands for αv integrins. In contrast to the experiments using F11, blocking monoclonal antibodies against the β1 (Ha2/5) and β5 (P1F6) integrin subunit had no effect on PDGF-induced proliferation on Vn (Figure 2A), showing that PDGF-induced OP proliferation on Vn is mediated by αvβ3 and not by αvβ1 or αvβ5.
Fig. 2. Identification of which integrin is responsible for PDGF-AA-mediated enhanced OP proliferation on Vn. (A) OPs were plated on Vn (10 µg/ml) and the ability of integrin-blocking antibodies and blocking RGD peptides to abolish the PDGF-AA (1 ng/ml)-mediated OP proliferation on Vn was determined, as described in Materials and methods. PDL represents the BrdU incorporation on PDL in the absence of any growth factor. Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (***P <0.001) between control (Vn + 1 ng/ml PDGF) and any other indicated condition. Note that the PDGF-AA-mediated enhanced OP proliferation on Vn is abolished by anti-integrin β3 and blocking RGD peptides. (B) OPs expressing vector only (VO), the dominant-negative IL2Rβ3 construct or the IL2Rβ1 construct were subjected to proliferation assays on PDL in the presence of 1 ng/ml PDGF-AA, as described in Materials and methods. No significant proliferation is observed in the absence of PDGF (data not shown). Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (*P <0.05, ***P <0.001) between VO and any other indicated condition. Note the decrease in proliferative capacity of OPs expressing the dominant-negative IL2Rβ3 construct and the increased proliferation of the dominant-negative IL2Rβ1-expressing OPs.
To confirm further that αvβ3 is required for oligodendrocyte proliferation at ≤1 ng/ml PDGF, we infected OPs with retroviral vectors expressing either a cytoplasmic β3 or β1 subunit attached to the extracellular and transmembrane domain of the interleukin-2 receptor (IL2Rβ3 and IL2Rβ1). Previous studies have shown that these IL2R–integrin constructs can act as dominant-negative inhibitors of integrin function (LaFlamme et al., 1994). For these experiments, designed to confirm integrin function, it was necessary to perform assays with OPs previously expanded using growth factors so as to allow G418 selection of cells expressing the IL2R–integrin chimera. However, as shown in Figure 2B, expression of IL2Rβ3 in OPs resulted in an inhibition of the proliferative response to ≤1 ng/ml PDGF when compared with vector only (VO) controls, so confirming the role of αvβ3 even in expanded cell populations. In contrast, expression of IL2Rβ1 resulted in an enhanced proliferative response to these PDGF concentrations, suggesting that β1 integrins function as inhibitors of OP proliferation. This result confirms that this dominant-negative approach using chimeras is specific for individual β subunits and does not cause trans-dominant inhibition of multiple β integrin subunits as has been described with soluble integrin-specific ligands (Diaz-Gonzalez et al., 1996). As expected, since the IL2R-constructs do not bind any ligand, these inhibitory or stimulatory effects were seen on all substrates, with similar results obtained when the progenitors were plated on either PDL, Ln-1 or Vn (data not shown).
PDGFαR and integrin αvβ3 co-associate in OP cells
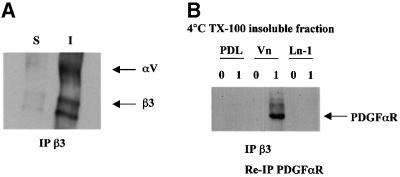
To examine the mechanisms of interaction between the PDGFαR and αvβ3 integrin, we next determined whether there is a physical association between these two receptors, as reported for the PDGFβR and αvβ3 integrin in other cell types (Schneller et al., 1997; Woodard et al., 1998; Borges et al., 2000). As shown in Figure 3A, immunoprecipitation of OP cell surface proteins identified by biotin labelling showed αvβ3 to be present only in the cellular protein fraction insoluble in Triton X-100 at 4°C. This result is consistent with our previous studies on oligodendroglial αv integrins, in which we did not observe αvβ3 integrin in the fraction soluble in Triton X-100 at 4°C until later stages of differentiation (Milner et al., 1997), and suggests that αvβ3 is normally associated with the cytoskeleton in OPs. In contrast, western blot analysis of Triton X-100 cell lysates revealed that most of the PDGFαR was present in the soluble fraction on all substrates (data not shown). To demonstrate if there was a physical link between a subfraction of the PDGFαR in the Triton X-100-insoluble fraction and αvβ3 integrin, anti-β3 antibody immunoprecipitates from the Triton X-100-insoluble fractions were re-immunoprecipitated with anti-PDGFαR antibodies. Immunoblotting under reducing conditions with anti-PDGFαR then showed an association of αvβ3 and the PDGFαR under those conditions that induce OP proliferation (Figure 3B). This associated PDGFαR could not be detected by the less sensitive technique of immunoblotting following the anti-β3 immunoprecipitation. This shows that only a small subfraction of the total PDGFαR was associated with the integrin, as expected from the western blotting experiments in which the integrin and most of the growth factor receptor were found in different fractions following Triton X-100 solubilization. However, the association was specific, as both engagement of the αvβ3 integrin and growth factor binding to the PDGFαR were essential, with no association seen by this double immunoprecipitation protocol in the absence of PDGF or on PDL substrates. Interestingly, at the high concentrations of PDGF (10 ng/ml) required for proliferation on Ln-1 or PDL substrates, no association of the PDGFαR and αvβ3 integrin was observed (data not shown). From these results, we therefore conclude that in the presence of PDGF at physiological concentrations and integrin ligand binding, an association of the PDGFαR with αvβ3 integrin is established in OPs.
Fig. 3. Association of integrin αvβ3 with the PDGFαR in Triton X-100-insoluble fractions. (A) Freshly purified OPs were plated on PDL-coated dishes for 1 h, surface labelled with biotin, scraped and extracted with 1% Triton X-100. Both soluble (S) and insoluble (I) fractions were immunoprecipitated with anti-β3 (F11) and analysed by SDS–PAGE/western blot (7.5% gels) followed by detection with streptavidin–peroxidase and ECL, as described in Materials and methods. Note the expression of β3 solely in the detergent-insoluble fractions. (B) OPs were plated on either PDL, Vn or Ln-1 for 30 min, and then stimulated with 0 or 1 ng/ml PGDF-AA (0 and 1, respectively) at 37°C for 5 min, lysed, extracted with 1% Triton X-100, and insoluble fractions immunoprecipitated with anti-β3. Anti-β3 immunoprecipitates were then reprecipitated with anti-PDGFαR, and analysed for PDGFαR under reducing conditions with SDS–PAGE/western blot (7.5% gels) using ECL detection, as described in Materials and methods. Note that an association between integrin αvβ3 and PDGFαR is observed on a Vn substrate in the presence of low levels of PDGF-AA.
Both phosphatidylinositol 3-kinase and PKC activity are required for αvβ3-mediated proliferation at low PDGF concentrations
To examine the molecular mechanism by which the interaction between the PDGFαR and αvβ3 integrin might potentiate proliferation, we next investigated the signalling pathway(s) involved using pharmacological compounds capable of inhibiting specific signalling molecules. As shown in Figure 4, αvβ3-mediated OP proliferation requires both a phosphatidylinositol 3-kinase (PI3K)- and a protein kinase C (PKC)-dependent signalling pathway. Wortmannin, a PI3K inhibitor (Arcaro and Wymann, 1993), and bisindolylmaleimide (BIM), a specific PKC inhibitor (Toullec et al., 1991), inhibited OP proliferation on Vn at ≤1 ng/ml PDGF. The pp70 S6 kinase down stream of PI3K was also involved, as a potent inhibitor (rapamycin; Price et al., 1992) blocked proliferation. In contrast, the mitogen-activated protein kinase (MAPK) signalling pathway was not involved in OP proliferation under these conditions since PD098059, a MEKK inhibitor (Dudley et al., 1995), was not able to counteract the enhanced proliferation (Figure 4). Autophosphorylation of the PDGFαR was necessary as pre-treatment of the OPs with AG1295, a specific inhibitor of the PDGFαR tyrosine kinase activated by autophosphorylation (Kovalenko et al., 1994), inhibited the PDGF-induced proliferative response on Vn.
Fig. 4. The effect of inhibition of different signal transduction pathways on the PDGF-mediated enhanced OP proliferation on Vn. OPs were either left untreated (ctrl) or were pre-exposed to PD098059 (PD, 50 µM), AG1295 (AG, 10 µM), wortmannin (WM, 50 nM), rapamycin (RM, 20 ng/ml) or BIM (0.5 µM) for 30 min at 37°C (in suspension), and subsequently plated on Vn (10 µg/ml). The ability of these specific signalling inhibitors to abolish the PDGF-AA (1 ng/ml)-mediated OP proliferation on Vn was determined as described in Materials and methods. PDL represents the BrdU incorporation on PDL in the absence of any growth factor. Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (***P <0.001) between control (Vn + 1 ng/ml PDGF) and any other indicated condition. Note that the incorporation of BrdU was significantly decreased when OPs were co-treated with AG1295 (PDGFαR tyrosine kinase inhibitor), wortmannin (PI3K inhibitor), rapamycin (pp70 S6 kinase inhibitor) or BIM (PKC inhibitor), but not with PD098059 (p42/p44 MAPK inhibitor).
PKC activation mimics PDGF exposure by enhancing oligodendrocyte proliferation on vitronectin in the absence of growth factor
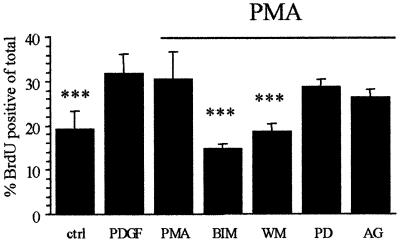
To determine whether the PKC- and PI3K-dependent signalling pathways required for OP proliferation on Vn at ≤1 ng/ml PDGF operate in a parallel or a sequential manner, the effect of PKC activation was examined. As shown in Figure 5, phorbol 12-myristate 13-acetate (PMA; a PKC activator) was able to mimic low PDGF concentrations by enhancing OP proliferation on Vn in the absence of PDGF. Similar to the response observed with PDGF, the PMA-stimulated proliferation was not observed when OPs were plated on either PDL or Ln-1, nor was it present if the cells were exposed to PMA prior to plating on Vn substrates (data not shown). The effect of PMA could be completely blocked by BIM and wortmannin, but not by AG1295 and PD098059 (Figure 5). Taken together with the observation that inhibiting either PKC or PI3K blocks proliferation on Vn at ≤1 ng/ml PDGF, these results using pharmacological inhibitors suggest first, that PKC and PI3K signalling operate sequentially and, secondly, that PKC activation is downstream of the PDGFαR but upstream of PI3K.
Fig. 5. Effect of PKC activation on the proliferative capacity of OPs on Vn. OPs were either left untreated (ctrl, PDGF, PMA) or were pre- exposed to PD098059 (PD, 50 µM), AG1295 (AG, 10 µM), wortmannin (WM, 50 nM) or BIM (0.5 µM) for 30 min at 37°C (in suspension), plated on Vn (10 µg/ml) and subsequently left untreated (ctrl) or treated with either 1 ng/ml PDGF (PDGF) or 100 nM PMA (all others, indicated by the horizontal bar), and the effect on proliferation was determined as described in Materials and methods. Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (***P <0.001) between PMA and the other indicated conditions. Note that PKC activation (in the absence of PDGF) via PMA mimicked the PDGF-mediated enhanced proliferation on Vn, and that inhibition of both PKC (BIM) and PI3K (WM) was able to abolish this enhanced proliferation.
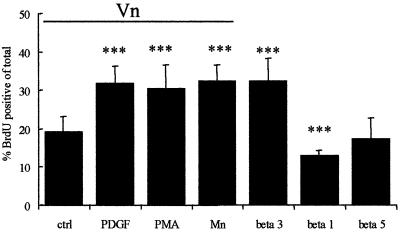
We also used an alternative method of establishing the relationship between αvβ3 and the PDGFαR, in which OPs were plated on the integrin function-blocking antibodies used as immobilized substrates. When presented in this way, antibodies can cluster integrins and hence initiate downstream signalling events (Miyamoto et al., 1995). Anti-β3 substrates were able to enhance OP proliferation in the absence of either PDGF or PMA (Figure 6), confirming that the integrin lies downstream of the PDGFαR in this signalling pathway. Importantly, this enhanced proliferation could be blocked with wortmannin but not PD098059 (data not shown). This shows that the same signalling pathway is involved as with PDGF and PMA, and also that the integrin is upstream of PI3K signalling for this particular response. As expected from the studies using either blocking antibodies or dominant-negative chimeras, no enhanced proliferative response was observed when the OPs were exposed to immobilized anti-β1 or anti-β5 (Figure 6). Indeed, the anti-β1 substrates resulted in a small but significant reduction in proliferation (Figure 6), consistent with the conclusion from the IL2R–integrin chimera data above that β1 integrins function as inhibitors of proliferation in OPs. Taken together with the experiments using PMA, we conclude from these data that ligand-induced PDGFαR signalling stimulates a PKC-dependent pathway, which in turn and in the presence of an integrin ligand leads to the induction of an αvβ3-dependent PI3K signalling pathway that directly stimulates OP proliferation.
Fig. 6. The effect of Mn2+ and immoblized integrin antibodies on the proliferative capacity of OPs on Vn. OPs were plated on Vn (indicated by the horizontal bar) or on immobilized integrin antibodies (β1, β3 and β5), and subsequently left untreated (ctrl, beta 1, beta 3 and beta 5), or treated with either 1 ng/ml PDGF (PDGF), 100 nM PMA (PMA) or 50 µM Mn2+ (Mn), and the effect on proliferation was determined as described in Materials and methods. Values shown are means ± SD of at least three independent experiments, each in duplicate. Statistical significance is shown (***P <0.001) between control (ctrl) and the other indicated conditions. Note that integrin activation via Mn2+ (in the presence of the ligand Vn) or plating on immobilized integrin β3 antibody (in the absence of ligand) mimicked the mitogenic response of OPs as observed with PDGF-AA or PMA on a Vn substrate.
PDGF-mediated activation of integrin αvβ3
The signalling sequence outlined above raises the question as to how PKC induces PI3K signalling in an integrin ligand-dependent manner. One potential mechanism is via affinity modulation, a mechanism for the activation of integrins (Hynes, 1992; Bazzoni and Hemler, 1998). For example, the αvβ3 integrin in quiescent endothelial cells is normally expressed in a low-affinity state for ligand binding and can be changed to a high affinity state via PKC signalling, presumably by conformational modification of the ligand-binding site resulting from inside-out signalling (Byzova and Plow, 1998). In this high affinity state and in the presence of ligand, the integrin could then initiate the downstream signalling pathways shown above to lead to proliferation. We therefore asked if other manipulations that activate αvβ3 integrin also induced OP proliferation on Vn substrates in the absence of PDGF. Mn2+ has been shown to activate αvβ3 integrin by increasing ligand affinity (Smith et al., 1994; Byzova and Plow, 1998). In keeping with a role for integrin activation in OP proliferation, Mn2+ induced proliferation of OPs already plated on Vn to a similar extent as PKC signalling or exposure to physiological levels of PDGF (Figure 6). As predicted, Mn2+-induced proliferation is dependent on αvβ3 ligand binding, as OP proliferation on PDL and Ln-1 was not observed in the presence of Mn2+ (data not shown).
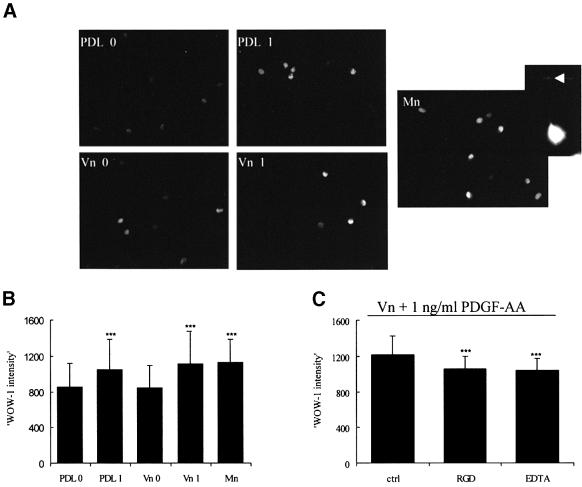
The experiments using Mn2+ or PMA show that activation (resulting from increased ligand affinity) of αvβ3 integrin is sufficient to induce proliferation in the absence of PDGF provided Vn is present, and therefore suggest that PDGF at physiological concentrations exerts a proliferative effect indirectly as a result of integrin activation. To confirm directly that PDGF increases the ligand affinity of αvβ3 in OPs, we used the ligand- mimetic antibody Fab, WOW-1. WOW-1 is a genetically engineered monovalent ligand that specifically recognizes a change in the affinity state of αvβ3 (Pampori et al., 1999). These experiments were performed on adherent cells, as primary OPs do not survive in suspension for the time span of the experiments. Positive control experiments using Mn2+ showed the expected increase in WOW-1 staining, as detected by immunofluorescence microscopy (Figure 7A and B). We next measured the intensity of WOW-1 staining in OPs in response to exposure to PDGF for 5 min. The cells were grown on either PDL or Vn, and assays were performed 1 h after plating. As shown in Figure 7B, 1 ng/ml PDGF was able to increase significantly the intensity of WOW-1 staining on both PDL and Vn, even though proliferation was only observed on the Vn substrate. While most of the WOW-1 binding was intracellular under these conditions (Figure 7A), with occasional detectable edge staining (Figure 7A, arrow), the intensity was significantly reduced by either RGD peptides or EDTA, and the degree of reduction was very similar to the increase seen in response to PDGF. These control experiments confirm that the change in WOW-1 binding in response to PDGF is indicative of activated integrins (Figure 7C). We conclude, therefore, that PDGF increases the ligand-binding affinity of integrin αvβ3 on both PDL and Vn.
Fig. 7. Effect of low levels of PDGF-AA on integrin αvβ3 activation. Freshly purified OPs were plated on PDL or Vn (10 µl) for 1 h, and either left untreated or exposed to either 1 ng/ml PDGF or 50 µM Mn2+ for 5 min at 37°C. OPs were fixed and permeabilized and stained with the activation-dependent monovalent antibody WOW-1, as described in Materials and methods. (A) Localization of high-affinity integrin αvβ3 in each of the conditions. Note that the WOW-1 staining is mainly intracellular, with occasional edge staining (arrow). (B) Quantification of the WOW-1 intensity in each condition within a single experiment, performed as described in Materials and methods. Statistical significance (***P <0.001) was seen in each of two experiments between 0 ng/ml PDGF (PDL 0) and 1 ng/ml PDGF (PDL 1), Vn + 1 ng/ml PDGF (Vn 1) and Mn2+ (Mn). Note that 1 ng/ml PDGF-AA is able to enhance WOW-1 binding intensity independently of the substrate. (C) Confirmation of the specificity of WOW-1 binding. RGD peptides (10 µg/ml) and EDTA (10 mM) were added 10 min before exposure to PDGF-AA. A single experiment is shown. Note the small but significant reduction in labelling intensity (***P <0.001, present in both experiments) between ctrl (Vn + 1 ng/ml PDGF) and the RGD- or EDTA-treated cells, reflecting the WOW-1 binding specific for activated integrins.
Discussion
The results presented here provide a novel mechanism for the regulation of precursor cell proliferation in the CNS (summarized in Figure 8). We have identified a specific interaction between the PDGFαR and the αvβ3 integrin in OPs and shown that proliferation at physiological PDGF-AA levels (0.1–1 ng/ml) requires αvβ3 ligand binding. Without such binding, as on Ln-1 and PDL substrates, much higher and non-physiological PDGF-AA levels (10 ng/ml) are needed to induce OP proliferation. The critical integrative mechanism involves PDGF-induced, PKC-dependent activation of αvβ3 integrin mediated by an increase in ligand affinity. This step is independent of any ECM substrate, but allows an αvβ3-mediated PI3K-dependent signalling pathway to promote proliferation in the presence of an appropriate ECM ligand. Previous work on the convergence of downstream signalling pathways activated by growth factors and integrins has emphasized the coordinating role of signalling molecules downstream of both the growth factor receptors and integrins (Schwartz and Assoian, 2001). The situation described here, in which the growth factor receptor signals via the integrin itself, shows similarities to the recently reported vascular endothelial growth factor (VEGF)- and EGF-mediated activation of integrin αvβ3 in endothelial cells (Byzova et al., 2000). The important consequence in both cell types is that growth factor-mediated integrin activation allows the integration of a long-range growth factor signal and a regional-dependent ECM signal, so allowing precise regulation of cell behaviour.

Fig. 8. Model for oligodendrocyte progenitor proliferation. As the levels of soluble growth factors in vivo are both limiting and too low to induce OP proliferation by themselves, local co-signals are needed to keep OPs proliferative and unable to differentiate prematurely. So, for example, by contacting integrin αvβ3 ligands, such as Vn on adjacent cells (neurons and astrocytes), a proliferative OP response can be induced at physiological PDGF concentrations. Upon both integrin αvβ3 engagement and PDGFαR ligand binding, the PDGFαR is sequestered to discrete detergent-insoluble membrane signalling platforms. In these membrane compartments, the PDGFαR is able to (further) activate integrin αvβ3 via a PKC-dependent signalling pathway. As a consequence, OP proliferation is induced via an integrin αvβ3-mediated PI3K-pp70 S6K-dependent signalling pathway. The fact that OP proliferation is dependent on the simultaneous action of a local, short-range signal (integrin ligand) and a more general, long-range signal (soluble growth factor) makes it possible for OP proliferation to be tightly regulated.
Integrin activation, as defined by an increased ability to bind ligand, can result from affinity modulation (as we have demonstrated here) and also from increased avidity associated with an enhanced ability of the integrins to diffuse and cluster within the membrane (Bazzoni and Hemler, 1998). Our observation that Mn2+ (which activates by stabilizing the high-affinity conformation of the integrin) promotes proliferation on Vn substrates in the absence of PDGF suggests that affinity modulation alone is sufficient for integrin-stimulated proliferation in OPs. However, avidity modulation could also be triggered by PDGFαR signalling in OPs. It has been shown in OPs that PDGF phosphorylates the PKC substrate MARCKS (Baron et al., 2000), and that PKC-induced phosphorylation leads to translocation of MARCKS into the cytosol and a redistribution of the cortical actin cytoskeleton (Baron et al., 1999). MARCKS has been implicated in the activation of β2 integrins by facilitating their release from cytoskeletal restraints and so allowing clustering (Zhou and Li, 2000), and could therefore play a similar role in the activation of OP integrins. Consistent with such a model, our experiments show that the PDGFαR is recruited into the Triton X-100 insoluble fraction (which contains the proteins associated with the cytoskeleton) in conditions that stimulate OP proliferation. This result suggests that rearrangements of the cytoskeletal association of signalling receptors occur alongside any increase in integrin affinity. However, our results do not address whether this is a primary event contributing to further integrin activation and signal amplification, or a secondary consequence of the integrin signalling induced by affinity modulation in response to PDGF that does not amplify the response further.
From a developmental standpoint, the model proposed in Figure 8 for the regulation of cell proliferation is important for two reasons. First, it emphasizes that proliferation requires both growth factor availability and the presence of the correct ECM, so allowing cells to behave differently from their immediate neighbours and set up patterns of proliferation even in microenvironments within which the growth factor concentration is uniform. In the case of OPs, at the physiological PDGF level, proliferation is absolutely dependent on the availability of an αvβ3 ligand. The αvβ3 integrin has been shown to bind several different ligands, including tenascin-C and Thy-1, both of which are present in the CNS (Morris, 1985; Joester and Faissner, 2001). Tenascin-C-deficient mice show decreased OP proliferation in vivo, in keeping with such a role for this ligand (Garcion et al., 2001). The recent finding that Thy-1 is a ligand for β3 integrin (Leyton et al., 2001) is potentially very interesting, as Thy-1 is a neuronal surface glycoprotein that rises 100-fold during early postnatal CNS development and is expressed on the axon surface once axonal growth is complete (Morris, 1985; Xue et al., 1991). Expression of Thy-1 on the axons and recognition by OP αvβ3 therefore provides a mechanism for bi-directional axo-glial signalling. This signalling will ensure that OP proliferation is precisely controlled and confined only to those cells that have established appropriate axonal contact (i.e. to those axons that have completed their growth and not yet myelinated) and have therefore migrated appropriately. Other possible αvβ3 integrin ligands in the CNS are L1 and ADAM23 (Montgomery et al., 1996; Yip et al., 1998; Sagane et al., 1999), but, as the binding of ADAM23 to αvβ3 is reported to be RGD independent, this ligand may elicit signalling responses different from those we have examined in this study (Cal et al., 2000). Secondly, our model in which integrin activation is a critical regulator of signalling provides a mechanism by which other molecules that regulate integrin activation can contribute to the regulation of OP behaviour. Potential examples are the Eph receptor family and their ligands, ephrins, as well as the Edg receptors, all of which have been shown to alter integrin activation levels in other cell types (Huynh-Do et al., 1999; Zou et al., 1999; Davy and Robbins, 2000; Huai and Drescher, 2001; Paik et al., 2001). Further studies are required to determine whether these signalling molecules also alter integrin activation in OPs and so provide a network of diverse cell–cell signalling systems, all of which are integrated by their role in integrin activation. Additionally, it will be important to determine whether (as we would predict) other aspects of OP development such as migration, for which we have demonstrated a role for the αvβ1 integrin (Milner et al., 1996), are also coordinated by integrin activation.
Our present results also have important implications for studies of the CNS response to injury, as they predict that short- or long-term changes in the ECM will alter precursor cell behaviour. Such changes have been described in models of CNS injury. For example, acute stab lesions have been shown to induce a transient increase in the proliferation of adjacent OPs (as defined by their expression of the NG2 molecule, which co-localizes with the well-defined PDGFαR marker of OPs in normal development; Nishiyama et al., 1996) adjacent to the lesion (Levine, 1994). The loss of the blood–brain barrier associated with these lesions will result in the entry into the CNS of serum proteins such as Fn and Vn, which will provide ligands for αv integrins. We suggest that these ECM changes will potentiate the effects of any endogenous or exogenous (serum-derived) mitogens present in this region and contribute to the increase in OP number at the site of injury. Equally, chronic lesions such as those seen in multiple sclerosis (MS) contain an altered ECM (Sobel, 1998). By altering the levels of ligand for αvβ3, this abnormal ECM may inhibit the differentiation of OPs into oligodendrocytes with the capacity to form new myelin and repair the lesion. This hypothesis would explain the presence of OPs in many MS lesions apparently unable to contribute to repair, and also makes the important prediction that therapeutic interventions designed solely to increase growth factor concentrations in the lesion will have little beneficial effect on repair.
Materials and methods
Reagents and antibodies
GRGDSP and GRGESP (control) hexapeptides were obtained from Life Technologies. PDGF-AA was obtained from Peprotech. All pharmacological signalling pathway inhibitors were obtained from Calbiochem-Novobiochem Corporation. All other chemicals, including all cell culture media, were purchased from Sigma Chemical Co., unless stated otherwise. Anti-integrin β3 (F11, mouse IgG1) (Helfrich et al., 1992) was kindly provided by Dr M.Horton, London, UK. WOW-1 was generated as previously described (Pampori et al., 1999). Anti-integrin β1 (Ha2/5, Hamster IgM) and anti-integrin β5 (P1F6, mouse IgG1) were supplied by PharMingen and Chemicon, respectively. The polyclonal antibody against the PDGFαR (C-20) was obtained from Santa Cruz. Linker antibodies for immunoprecipitation and coating were obtained from Nordic Immunological Laboratories.
IL2R constructs
IL2Rβ1 and IL2Rβ3 cDNAs were kind gifts of Dr S.E.LaFlamme, New York, NY. The cDNAs were cloned into a retroviral vector (pLIXN, Clontech Laboratories). Retroviral infection was then performed as described previously (Relvas et al., 2001); briefly, freshly purified OPs were kept proliferative during the infection by growing them in SATO medium containing PDGF-AA (10 ng/ml) and FGF-2 (10 ng/ml). After retroviral infection, the cells were kept under selection (G418) for 5 days, trypsinized, resuspended in DMEM, replated and their mitogenic response analysed as described below under ‘Proliferation studies’.
Cell culture
Primary mixed brain cell cultures cells were prepared from forebrains of 1- to 2-day-old Sprague–Dawley rats, and OPs were isolated by mechanical dissociation, followed by differential adhesion, as described previously (Milner and ffrench-Constant, 1994). Enriched OPs were resuspended in DMEM supplemented with 2 mM glutamine and penicillin/streptomycin (DMEM), and treated as indicated.
Proliferation studies
Cell proliferation was measured by determining the incorporation of the thymidine analogue BrdU. Assays were performed in minimal medium (DMEM) so as to minimize the presence of other possible signalling factors, such as growth factors and ECM substrates. Eight-well permanox Lab-Tek chamber slides (Nalge Nunc Int.) were coated for at least 4 h at 37°C with either PDL, Vn or Ln-1 (all at 10 µg/ml). When the cells were plated on immobilized integrin antibodies, the chamber slides were coated for 2 h at 37°C with a linker antibody [10 µg/ml in phosphate-buffered saline (PBS)], followed by a 2 h coating at 37°C with the indicated integrin antibodies (10 µg/ml in PBS). Non-specific binding was blocked with heat-inactivated bovine serum albumin (BSA; ≥30 min at 37°C). Freshly purified OPs were suspended in DMEM and kept in suspension at 37°C for 30 min so as to minimize activation of integrin and growth factor signalling pathways. A total of 20 000–40 000 cells were then added to each well, and growth factors, PMA or Mn2+ added 2–5 min after plating at the indicated concentrations. For the experiments with the different signalling inhibitors, the OPs were pre-treated with these agents during the 30 min in suspension and they were also present during the time span of the experiment (16 h). The concentrations of the signalling inhibitors used were: 50 µM PD098059, 0.5 µM BIM, 20 ng/ml rapamycin, 10 µM AG1295 and 50 nM wortmannin. The agents were solubilized in cell culture dimethylsulfoxide (DMSO), with the final concentration of DMSO in the medium being ≤0.1%. Controls contained the same DMSO concentration. Function blocking integrin antibodies (10 µg/ml) and peptides (10 µg/ml) were added immediately after plating and before PDGF-AA was added. Cultures were incubated with 10 µM BrdU for 16 h (overnight). BrdU incorporation was detected using a BrdU proliferation detection kit (Roche) according to the manufacturer’s instructions. Cells were mounted in ImmunoFloure (ICN) to prevent image fading, and subsequently were examined with a Zeiss fluorescence microscope. Data represent the results of at least three independent experiments, each performed in duplicate with at least 500 cells per well counted. Statistical analysis was performed using the unpaired Student’s t-test (statistical significance was accepted for *P <0.05, **P < 0.01 and ***P <0.001).
Immunoprecipitations and western blotting
Cells were treated as indicated, washed twice with PBS, scraped and cell pellets lysed in lysis buffer [50 mM Tris–HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml pepstatin A, 2 µg/m aprotinin, 5 µg/ml leupeptin, 2 mM sodium fluoride, 2 mM sodium vanadate and 1 mM sodium pyrophosphate pH 7.4] on ice for 30 min. For cell surface labelling experiments, cell surface molecules were labelled with 0.1 mg/ml NHS-LC-biotin (Pierce) for 25–30 min at 37°C, washed three times in cell wash buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM CaCl2 and 1 mM MgCl2 pH 7.5), scraped and lysed as described above. Extracts, i.e. supernatant and pellet, were collected by centrifugation at 14 000 r.p.m. for 10 min at 4°C. The insoluble pellet was washed once with lysis buffer and solubilized in a small volume of solubilization buffer (50 mM Tris–HCl, 5 mM EDTA, 1% SDS) by passage through a 21-gauge needle, and diluted with extraction buffer to the same volume as the supernatant. Supernatants were also adjusted to an SDS concentration equal to that of the solubilized pellets. The amount of total protein in the extracts was determined with the Bio-Rad detergent-compatible protein assay with BSA as standard. As indicated, equal amounts of protein or equal volumes were then subjected to immunoprecipitation. Immunoprecipitations with anti-β3 (F11, 1:250) were carried out overnight with 40 µl of rabbit-anti-mouse pre-linked protein A–Sepharose (Pharmacia) at 4°C. The beads were washed extensively four times with immunoprecipitation wash buffer (cell wash buffer + 0.5 M NaCl and 1% NP-40) and once with PBS. If identification of total integrin β3 was required, precipitated biotin-labelled cell surface integrin β3 was analysed by SDS–PAGE (7.5%) under non-reducing conditions, followed by immunoblot ECL detection with streptavidin– peroxidase (Amersham). A sequential immunoprecipitation technique was used to analyse the association between αvβ3 and the PDGFαR. The immune complexes (beads) from the anti-β3 immunoprecipitation were resuspended in 25 µl of 50 mM Tris–HCl (pH 6.8) supplemented with 2% SDS and heated for 5 min at 95°C. Supernatants were then diluted 10-fold with lysis buffer and re-immunoprecipitated with anti-PDGFαR (1:100) as described above for β3. Precipitated PDGFαR was visualized by SDS–PAGE (7.5%) under reducing conditions, followed by western blotting with anti-PDGFαR and ECL detection. For western blotting, proteins were separated by SDS–PAGE and electroblotted onto nitrocellulose membranes (Hybond-C, Pharmacia). Membranes were blocked in either 4% BSA (integrin) or 10% non-fat dry milk (PDGFαR) in Tris-buffered saline (TBS) for 1 h at room temperature or overnight at 4°C. Blots were incubated with primary antibody overnight (anti-PDGFαR 1:400) at 4°C in 1% milk in TBS containing 0.1% Tween-20 (TBS-T), followed by a 2 h incubation with the appropriate secondary peroxidase-conjugated antibody (Amersham) in TBS-T. The immunoreactive proteins were visualized using ECL according to the manufacturer’s instructions (Amersham).
WOW-1 experiments
Enriched OPs were resuspended in DMEM, and 40 000 cells were plated onto each well of a Vn- or PDL-pre-coated/BSA blocked (see ‘Proliferation studies’) 8-well permanox Lab-Tek chamber slide. After 1 h, adherent OPs were either left untreated or treated with PDGF-AA (1 ng/ml) or Mn2+ (50 µM) for 5 min. For the WOW-1 specificity studies, RGD peptides (10 µg/ml) or EDTA (10 mM) were added 10 min before addition of PDGF-AA. Cells were fixed with 2% paraformaldehde in PBS for 15 min, followed by 4% paraformaldehyde for 20 min. Cells were permeabilized and blocked with 0.1% Triton X-100 and 10% normal goat serum (NGS) and incubated with WOW-1 (25 µg/ml in 1% NGS in PBS) overnight at 4°C. After three washes with PBS, cells were next incubated for 30 min with Alexa Fluor 488 goat anti-mouse IgG (H+L) (Molecular Probes). Cells were mounted in ImmunoFloure (ICN) to prevent image fading, and WOW-1 labelling intensity was analysed and evaluated using image analysis on a Zeiss fluorescence microscope with Openlab software (Improvision). Images of cells grown under the different conditions on a single slide and then immunolabelled together were obtained using a Hamamatsu C4742-95 camera. Individual cells were then selected using a lasso function and the average pixel intensity obtained using a 12-bit scale giving arbitrary values between 0 and 4096. The mean intensity of at least 60 individual cells per condition was determined in two independent experiments. Statistical analysis was performed using the unpaired Student’s t-test, with statistical significance calculated within each experiment and shown as *P <0.05, **P < 0.01 and ***P <0.001 when present in all experiments.
Acknowledgments
Acknowledgements
We wish to thank Dr Susan LaFlamme for providing the IL2R chimeras, Dr Joao Relvas for preparing retroviral vectors containing these chimeras, and Dr Joel Levine and members of the ffrench-Constant laboratory for helpful discussions. This work was supported by grants from the Stichting Vrienden MS Research (Dutch Foundation for the Support of MS Research) to W.B., the Wellcome Trust (Sir Henry Wellcome Commemorative award scheme) and NIH grant HL56595 to S.J.S. C.ff.-C. holds a Wellcome Trust Research Leave Fellowship for Clinical Academics.
References
- Adams J.C. and Watt,F.M. (1993) Regulation of development and differentiation by the extracellular matrix. Development, 117, 1183–1198. [DOI] [PubMed] [Google Scholar]
- Arcaro A. and Wymann,M.P. (1993) Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phospha tidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J., 296, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong R.C., Harvath,L. and Dubois-Dalcq,M.E. (1990) Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J. Neurosci. Res., 27, 400–407. [DOI] [PubMed] [Google Scholar]
- Bansal R. and Pfeiffer,S.E. (1997) Regulation of oligodendrocyte differentiation by fibroblast growth factors. Adv. Exp. Med. Biol., 429, 69–77. [DOI] [PubMed] [Google Scholar]
- Baron W., de Vries,E.J., de Vries,H. and Hoekstra,D. (1999) Protein kinase C prevents oligodendrocyte differentiation: modulation of actin cytoskeleton and cognate polarized membrane traffic. J. Neurobiol., 41, 385–398. [DOI] [PubMed] [Google Scholar]
- Baron W., Metz,B., Bansal,R., Hoekstra,D. and de Vries,H. (2000) PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol. Cell. Neurosci., 15, 314–329. [DOI] [PubMed] [Google Scholar]
- Barres B.A. and Raff,M.C. (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron, 12, 935–942. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. and Hemler,M.E. (1998) Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci., 23, 30–34. [DOI] [PubMed] [Google Scholar]
- Blaschuk K.L., Frost,E.E. and ffrench-Constant,C. (2000) The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by αV integrins. Development, 127, 1961–1969. [DOI] [PubMed] [Google Scholar]
- Bogler O., Wren,D., Barnett,S.C., Land,H. and Noble,M. (1990) Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc. Natl Acad. Sci. USA, 87, 6368–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges E., Jan,Y. and Ruoslahti,E. (2000) Platelet-derived growth factor receptor β and vascular endothelial growth factor receptor 2 bind to the β3 integrin through its extracellular domain. J. Biol. Chem., 275, 39867–39873. [DOI] [PubMed] [Google Scholar]
- Butt A.M. and Berry,M. (2000) Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum. J. Neurosci. Res., 59, 477–488. [DOI] [PubMed] [Google Scholar]
- Byzova T.V. and Plow,E.F. (1998) Activation of αVβ3 on vascular cells controls recognition of prothrombin. J. Cell Biol., 143, 2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzova T.V., Goldman,C.K., Pampori,N., Thomas,K.A., Bett,A., Shattil,S.J. and Plow,E.F. (2000) A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell, 6, 851–860. [PubMed] [Google Scholar]
- Cal S., Freije,J.M., Lopez,J.M., Takada,Y. and Lopez-Otin,C. (2000) ADAM 23/MDC3, a human disintegrin that promotes cell adhesion via interaction with the αvβ3 integrin through an RGD-independent mechanism. Mol. Biol. Cell, 11, 1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll P.D., Musacchio,J.M., Hardy,R., Reynolds,R., Marchionni,M.A. and Salzer,J.L. (1996) GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron, 17, 229–243. [DOI] [PubMed] [Google Scholar]
- Davy A. and Robbins,S.M. (2000) Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J., 19, 5396–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone D.W. (1994) Adhesion and matrix in vertebrate development. Curr. Opin. Cell Biol., 6, 747–751. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez F., Forsyth,J., Steiner,B. and Ginsberg,M.H. (1996) Trans-dominant inhibition of integrin function. Mol. Biol. Cell., 7, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley D.T., Pang,L., Decker,S.J., Bridges,A.J. and Saltiel,A.R. (1995) A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl Acad. Sci. USA, 92, 7686–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M. et al. (1999) Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development, 126, 457–467. [DOI] [PubMed] [Google Scholar]
- Garcion E., Faissner,A. and ffrench-Constant,C. (2001) Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development, 128, 2485–2496. [DOI] [PubMed] [Google Scholar]
- Helfrich M.H., Nesbitt,S.A. and Horton,M.A. (1992) Integrins on rat osteoclasts: characterization of two monoclonal antibodies (F4 and F11) to rat β3. J. Bone Miner. Res., 7, 345–351. [DOI] [PubMed] [Google Scholar]
- Huai J. and Drescher,U. (2001) An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem., 276, 6689–6694. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U., Stein,E., Lane,A.A., Liu,H., Cerretti,D.P. and Daniel,T.O. (1999) Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through αvβ3 and α5β1 integrins. EMBO J., 18, 2165–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1994) Genetic analyses of cell–matrix interactions in development. Curr. Opin. Genet. Dev., 4, 569–574. [DOI] [PubMed] [Google Scholar]
- Joester A. and Faissner,A. (2001) The structure and function of tenascins in the nervous system. Matrix Biol., 20, 13–22. [DOI] [PubMed] [Google Scholar]
- Kondo T. and Raff,M. (2000) Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science, 289, 1754–1757. [DOI] [PubMed] [Google Scholar]
- Kovalenko M., Gazit,A., Bohmer,A., Rorsman,C., Ronnstrand,L., Heldin,C.H., Waltenberger,J., Bohmer,F.D. and Levitzki,A. (1994) Selective platelet-derived growth factor receptor kinase blockers reverse cis-transformation. Cancer Res., 54, 6106–6114. [PubMed] [Google Scholar]
- LaFlamme S.E., Thomas,L.A., Yamada,S.S. and Yamada,K.M. (1994) Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration and matrix assembly. J. Cell Biol., 126, 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.M. (1994) Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J. Neurosci., 14, 4716–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton L., Schneider,P., Labra,C.V., Ruegg,C., Hetz,C.A., Quest,A.F. and Bron,C. (2001) Thy-1 binds to integrin β3 on astrocytes and triggers formation of focal contact sites. Curr. Biol., 11, 1028–1038. [DOI] [PubMed] [Google Scholar]
- McCarthy K.D. and de Vellis,J. (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol., 85, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F.A. and Dubois-Dalcq,M. (1988) Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J. Neurosci. Res., 21, 199–209. [DOI] [PubMed] [Google Scholar]
- Milner R. and ffrench-Constant,C. (1994) A developmental analysis of oligodendroglial integrins in primary cells: changes in αv-associated β subunits during differentiation. Development, 120, 3497–3506. [DOI] [PubMed] [Google Scholar]
- Milner R., Edwards,G., Streuli,C. and ffrench-Constant,C. (1996) A role in migration for the αVβ1 integrin expressed on oligodendrocyte precursors. J. Neurosci., 16, 7240–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R., Frost,E., Nishimura,S., Delcommenne,M., Streuli,C., Pytela,R. and ffrench-Constant,C. (1997) Expression of αvβ3 and αvβ8 integrins during oligodendrocyte precursor differentiation in the presence and absence of axons. Glia, 21, 350–360. [PubMed] [Google Scholar]
- Miyamoto S., Teramoto,H., Coso,O.A., Gutkind,J.S., Burbelo,P.D., Akiyama,S.K. and Yamada,K.M. (1995) Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol., 131, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery A.M., Becker,J.C., Siu,C.H., Lemmon,V.P., Cheresh,D.A., Pancook,J.D., Zhao,X. and Reisfeld,R.A. (1996) Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J. Cell Biol., 132, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. (1985) Thy-1 in developing nervous tissue. Dev. Neurosci., 7, 133–160. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Lin,X.H., Giese,N., Heldin,C.H. and Stallcup,W.B. (1996) Co-localization of NG2 proteoglycan and PDGFα-receptor on O2A progenitor cells in the developing rat brain. J. Neurosci. Res., 43, 299–314. [DOI] [PubMed] [Google Scholar]
- Osterhout D.J., Ebner,S., Xu,J., Ornitz,D.M., Zazanis,G.A. and McKinnon,R.D. (1997) Transplanted oligodendrocyte progenitor cells expressing a dominant-negative FGF receptor transgene fail to migrate in vivo. J. Neurosci., 17, 9122–9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J.H., Chae,S., Lee,M.J., Thangada,S. and Hla,T. (2001) Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J. Biol. Chem., 276, 11830–11837. [DOI] [PubMed] [Google Scholar]
- Pampori N., Hato,T., Stupack,D.G., Aidoudi,S., Cheresh,D.A., Nemerow,G.R. and Shattil,S.J. (1999) Mechanisms and consequences of affinity modulation of integrin αVβ3 detected with a novel patch-engineered monovalent ligand. J. Biol. Chem., 274, 21609–21616. [DOI] [PubMed] [Google Scholar]
- Price D.J., Grove,J.R., Calvo,V., Avruch,J. and Bierer,B.E. (1992) Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science, 257, 973–977. [DOI] [PubMed] [Google Scholar]
- Pringle N., Collarini,E.J., Mosley,M.J., Heldin,C.H., Westermark,B. and Richardson,W.D. (1989) PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J., 8, 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relvas J.B., Setzu,A., Baron,W., Buttery,P.C., LaFlamme,S.E., Franklin,R.J. and ffrench-Constant,C. (2001) Expression of dominant-negative and chimeric subunits reveals an essential role for β1 integrin during myelination. Curr. Biol., 11, 1039–1043. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A. and Weiss,S. (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science, 255, 1707–1710. [DOI] [PubMed] [Google Scholar]
- Richardson W.D., Pringle,N., Mosley,M.J., Westermark,B. and Dubois-Dalcq,M. (1988) A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell, 53, 309–319. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. (1996) RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol., 12, 697–715. [DOI] [PubMed] [Google Scholar]
- Sagane K., Yamazaki,K., Mizui,Y. and Tanaka,I. (1999) Cloning and chromosomal mapping of mouse ADAM11, ADAM22 and ADAM23. Gene, 236, 79–86. [DOI] [PubMed] [Google Scholar]
- Schneller M., Vuori,K. and Ruoslahti,E. (1997) αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J., 16, 5600–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A. and Assoian,R.K. (2001) Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci., 114, 2553–2560. [DOI] [PubMed] [Google Scholar]
- Smith J.W., Piotrowicz,R.S. and Mathis,D. (1994) A mechanism for divalent cation regulation of β3-integrins. J. Biol. Chem., 269, 960–967. [PubMed] [Google Scholar]
- Sobel R.A. (1998) The extracellular matrix in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol., 57, 205–217. [DOI] [PubMed] [Google Scholar]
- Temple S. and Raff,M.C. (1985) Differentiation of a bipotential glial progenitor cell in a single cell microculture. Nature, 313, 223–225. [DOI] [PubMed] [Google Scholar]
- Toullec D. et al. (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem., 266, 15771–15781. [PubMed] [Google Scholar]
- Tropepe V., Sibilia,M., Ciruna,B.G., Rossant,J., Wagner,E.F. and van der Kooy,D. (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol., 208, 166–188. [DOI] [PubMed] [Google Scholar]
- van Heyningen P., Calver,A.R. and Richardson,W.D. (2001) Control of progenitor cell number by mitogen supply and demand. Curr. Biol., 11, 232–241. [DOI] [PubMed] [Google Scholar]
- Wang S., Sdrulla,A.D., diSibio,G., Bush,G., Nofziger,D., Hicks,C., Weinmaster,G. and Barres,B.A. (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron, 21, 63–75. [DOI] [PubMed] [Google Scholar]
- Woodard A.S., Garcia-Cardena,G., Leong,M., Madri,J.A., Sessa,W.C. and Languino,L.R. (1998) The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J. Cell Sci., 111, 469–478. [DOI] [PubMed] [Google Scholar]
- Xue G.P., Rivero,B.P. and Morris,R.J. (1991) The surface glycoprotein Thy-1 is excluded from growing axons during development: a study of the expression of Thy-1 during axogenesis in hippocampus and hindbrain. Development, 112, 161–176. [DOI] [PubMed] [Google Scholar]
- Yip P.M., Zhao,X., Montgomery,A.M. and Siu,C.H. (1998) The Arg–Gly–Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the αvβ3 integrin. Mol. Biol. Cell, 9, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. and Li,J. (2000) Macrophage-enriched myristoylated alanine-rich C kinase substrate and its phosphorylation is required for the phorbol ester-stimulated diffusion of β2 integrin molecules. J. Biol. Chem., 275, 20217–20222. [DOI] [PubMed] [Google Scholar]
- Zou J.X., Wang,B., Kalo,M.S., Zisch,A.H., Pasquale,E.B. and Ruoslahti,E. (1999) An Eph receptor regulates integrin activity through R-Ras. Proc. Natl Acad. Sci. USA, 96, 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]