Abstract
To study the role of caspase-6 during nuclear disassembly, we generated a chicken DT40 cell line in which both alleles of the caspase-6 gene were disrupted. No obvious morphological differences were observed in the apoptotic process in caspase-6- deficient cells compared with the wild type. However, examination of apoptosis in a cell-free system revealed a block in chromatin condensation and apoptotic body formation when nuclei from HeLa cells expressing lamin A or lamin A-transfected Jurkat cells were incubated in caspase-6-deficient apoptotic extracts. Transfection of exogenous caspase-6 into the clone reversed this phenotype. Lamins A and C, which are caspase-6-only substrates, were cleaved by the wild-type and heterozygous apoptotic extracts but not by the extracts lacking caspase-6. Furthermore, the caspase-6 inhibitor z-VEID-fmk mimicked the effects of caspase-6 deficiency and prevented the cleavage of lamin A. Taken together, these observations indicate that caspase-6 activity is essential for lamin A cleavage and that when lamin A is present it must be cleaved in order for the chromosomal DNA to undergo complete condensation during apoptotic execution.
Keywords: apoptosis/caspase-6/chromatin condensation/DT40/lamins
Introduction
The execution phase of apoptosis requires the activation of a family of highly specific proteases called caspases (Cohen, 1997; Thornberry, 1998; Earnshaw et al., 1999). Fourteen caspases have been identified in mammals; those implicated in apoptosis have been divided into two distinct subfamilies. The initiator caspase subfamily members transduce various signals into protease activity, thereby initiating a caspase cascade that proceeds through activation of the downstream effector or executioner caspases (for reviews see Budihardjo et al., 1999; Earnshaw et al., 1999; Hengartner, 2000).
The activated effector caspases-3, -6 and -7 are responsible for cleaving a number of target polypeptides, leading to the ultimate destruction of the cell. The role of caspase-3 in apoptotic execution has been widely studied, and many of its substrates discovered, starting with poly(ADP-ribose) polymerase (PARP-1) (Lazebnik et al., 1994; Earnshaw et al., 1999). Genetic analysis of caspase-3 function in mice has revealed that the enzyme is required for nuclear disassembly in drug-induced apoptosis (Kuida et al., 1996; Woo et al., 1998). Caspase-3 also appears to be responsible for the release of active CAD/DFF40, a DNase involved in DNA fragmentation and chromatin condensation, by cleaving its inhibitor ICAD (Liu et al., 1997; Enari et al., 1998).
The specific role played by caspase-6 is much less well understood. In some studies, caspase-6 maturation has been reported to depend on caspase-3 activation (Srinivasula et al., 1996; Slee et al., 2001). However, in other studies, caspase-6 activation occurred without caspase-3 activation (Miyashita et al., 1998) or before caspase-3 activation (Allsopp et al., 2000). The first substrates identified for this enzyme were the nuclear lamins (Orth et al., 1996; Takahashi et al., 1996a), and its target recognition sequence was identified as VEID (Takahashi et al., 1996a). Both chemical inhibitor studies and a follow-up study in which caspase-6 activity was inhibited selectively with the serpin SPI-2 suggested that lamin cleavage might be required for nuclear disassembly during apoptotic execution (Lazebnik et al., 1995; Takahashi et al., 1996b). Other substrates reported to be cleaved by caspase-6 include cytokeratin 18 (Caulin et al., 1997), focal adhesion kinase (Gervais et al., 1998), nuclear mitotic apparatus protein (NuMA) (Hirata et al., 1998), the β-amyloid precursor protein (Pellegrini et al., 1999), topoisomerase I (Samejima et al., 1999), nuclear matrix protein SATB1 (Galande et al., 2001), transcription factor AP-2α (Nyormoi et al., 2001), vimentin (Byun et al., 2001) and huntingtin (Wellington et al., 2000). In general, these substrates are also cleaved by other caspases, typically caspase-3, at other cleavage sites. In fact, the only substrate presently thought to be cleaved exclusively by caspase-6 is lamin A/C (Takahashi et al., 1996a; Slee et al., 2001).
Nuclear lamins are intermediate filament proteins that are the major components of the nuclear lamina. Lamins are classified into two subgroups. A-type lamins, including lamins A and C, are alternatively spliced products of a single gene locus. Lamins B1 and B2 are encoded by distinct genes (Franke, 1987). B-type lamins are expressed ubiquitously, whereas A-type lamins are absent from some non-terminally differentiated cells (Benavente et al., 1985; Guilly et al., 1987; Kaufmann, 1989; Lin and Worman, 1997). Lamins have been implicated in various nuclear functions, including the maintenance of chromatin organization and in DNA replication (see Discussion). Naturally occurring lamin A/C mutations have been implicated in the pathogenesis of certain forms of cardiac failure, muscular dystrophy, certain forms of lipodystrophy (Bonne et al., 1999; Flier, 2000; Shackleton et al., 2000) and in nuclear envelope defects (Raharjo et al., 2001; Vigouroux et al., 2001), suggesting that lamin A/C has additional functions that remain to be elucidated more fully.
Lamin cleavage appears to be an important event in the nuclear apoptotic process. Expression of uncleavable mutant lamin A or B caused significant delays in the onset of chromatin condensation and nuclear shrinkage during apoptosis (Rao et al., 1996). In addition, the onset of DNA fragmentation was also delayed in those cells, suggesting the unexpected possibility of a functional link between the apoptotic DNA fragmentation nuclease CAD/DFF40 (Enari et al., 1998; Samejima et al., 2001) and the nuclear lamina. Ultimately, however, the final stages of apoptosis appeared normal, including formation of apoptotic bodies in cells expressing uncleavable mutant lamins (Rao et al., 1996).
Here, we report the isolation of chicken DT40 B-lymphoma cell clones in which the caspase-6 gene has been deleted. We have investigated the consequence of caspase-6 deficiency on nuclear apoptotic processes in situ and in a cell-free system. We demonstrate that in cells that express lamin A, caspase-6 is required for the completion of chromatin condensation and formation of apoptotic bodies during apoptosis.
Results
Caspase-6 gene disruption
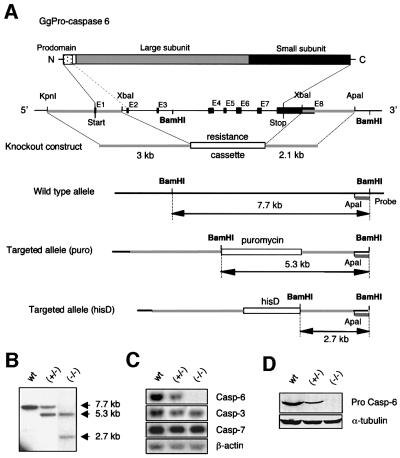
A chicken caspase-6 cDNA probe was used to isolate phage clones containing the caspase-6 locus from a DT40 genomic library. The entire locus was sequenced (DDBJ/EMBL/GenBank accession No. AF 469049) and the position of the exons determined by comparison with the cDNA sequence. The gene appears to be at least 8800 bp in length and contains eight exons (see Figure 1A). To disrupt the caspase-6 gene, we constructed a targeting vector in which a resistance cassette (puromycin or histidinol) was flanked by a 5′ genomic arm situated upstream of exon 2 and a 3′ genomic arm situated downstream of the stop codon (Figure 1A). Targeted integration of these constructs Casp6puro and Casp6his removes a 7211 bp gene fragment containing the majority of the open reading frame (888 bp out of 915 bp) and part of the 3′-untranslated region. Following insertion of these vectors, only the first nine amino acids of the enzyme prodomain could potentially be expressed, giving a peptide very unlikely to be functional. The deletion was performed by homologous recombination in the chicken lymphoma B-cell line DT40. Targeted events were recognized by Southern blot analysis of BamHI digestion of DNA and the use of a 3′ external probe. The probe recognizes a 7.7 kb band corresponding to the wild-type allele and a 5.3 or 2.7 kb band after targeted integration of the Casp6puro or Casp6his constructs, respectively (Figure 1B). Furthermore, targeted events were verified by Southern blot analysis using an external genomic 5′ probe (data not shown).
Fig. 1. Structure and targeting of the Gallus gallus caspase-6 gene. (A) Structure of the chicken caspase-6 gene together with the targeting vectors and homologous recombinants containing either the puromycin or the histidinol cassette. (B–D) Analysis of the caspase-6 homozygous (wt), heterozygous (+/–) and null clones (–/–). (B) Southern blot analysis of DNA digested by BamHI, using the 3′ genomic external probe ApaI–BamHI represented in (A). (C) Northern blot analysis showing chicken caspase-6, -3 and -7 mRNA expression. Note the loss of caspase-6 mRNA in the null clone. (D) Immunoblotting of caspase-6 using a polyclonal antibody directed against the large subunit of the enzyme (R549).
Generation of caspase-6-deficient DT40 clones
Wild-type DT40 cells were transfected with the Casp6puro construct and puromycin-resistant clones were analysed by Southern blotting in order to identify clones heterozygous for caspase-6. One of these heterozygous clones was then transfected with the Casp6his construct to delete the second allele. The targeting efficiency for the first allele was 8% and similar for both knockout constructs. The targeting of the second allele was more challenging. In the first experiment, only one targeted clone, out of 350 clones tested by Southern blotting, was found. In this clone, loss of caspase-6 expression was confirmed by northern blotting (Figure 1C) and by immunoblotting analysis (Figure 1D). Very different results were obtained subsequently when caspase-6+/– heterozygotes were transfected with a cDNA expressing caspase-6 prior to the second allele targeting event. In this case, an average of 4% target ing efficiency was observed and six independent caspase-6–/– clones were obtained. These targeting statistics initially suggested that caspase-6 might be essential for survival; however, subsequent experiments ruled this out conclusively.
Caspase-6-deficient DT40 cells are phenotypically normal
Caspase-6–/– cells showed a proliferation rate similar to wild-type DT40 cells (data not shown). The expression of the other effector caspases-3 and -7 in the caspase-6-deficient cells, assayed by northern blot analysis, appeared to be similar to the wild type, showing that disruption of the caspase-6 gene does not affect caspase-3 and -7 expression (Figure 1C).
When compared with wild-type DT40 cells, caspase-6–/– cells appeared to undergo apoptosis normally following exposure to etoposide (Figure 2). No differences in the timing or extent of chromatin condensation (Figure 2A) or in the fragmentation of DNA into a nucleosomal ladder (Figure 2B) could be observed over a 5 h time course. Similar results were obtained when wild-type and caspase-6–/– cells were induced to undergo staurosporine-induced apoptosis (data not shown).
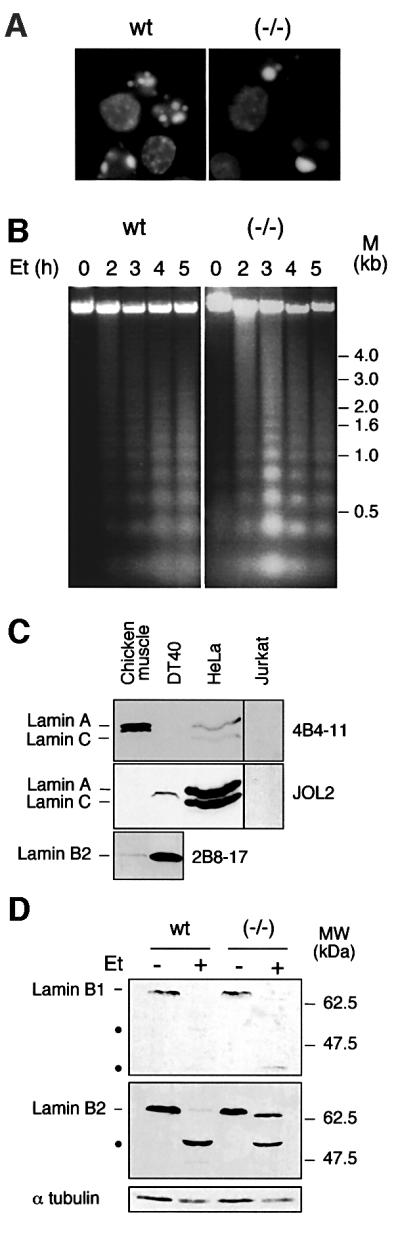
Fig. 2. Apoptosis in caspase-6-deficient DT40 cells is phenotypically normal. (A) DAPI staining of wild-type and caspase-6–/– cells treated with 10 µM etoposide for 4 h. Apoptosis was quantified by TUNEL labelling in this experiment, showing 58% apoptotic cells in the wild-type and 35% in the caspase-6–/– cells; no difference was observed when cells were treated with staurosporine (data not shown). (B) DNA fragmentation during the etoposide time course on wild-type and caspase-6–/– cells. (C) Immunoblotting analysis of DT40, Jurkat and HeLa whole-cell lysates together with chicken muscle tissue lysate, using monoclonal antibodies to human lamin A/C (JOL2), chicken lamin A (4B4-11) or chicken lamin B2 (2B8-17). This last antibody does not recognize the human form of lamin B2. The signal in the right lanes (Jurkat) was enhanced relative to that in the left lanes. (D) Immunoblotting analysis of wild-type DT40 and caspase-6–/– cells treated or not by etoposide at 10 µM for 4 h, using monoclonal antibodies to chicken lamin B1 and B2 (clone L-5 and 2B8-17, respectively). The cleavage products are shown by black dots.
Lamins are the best known substrates for caspase-6, especially lamin A, which so far is thought to be cleaved only by this caspase. Many lymphoid cells, however, lack lamin A (Guilly et al., 1987; Kaufmann, 1989), providing a potential explanation for the lack of an effect on nuclear apoptosis when caspase-6 was deleted in DT40 cells (a B-lymphocyte-derived cell line). Consistent with this possibility, we failed to detect the expression of lamin A in DT40 cells by using a monoclonal anti-chicken lamin A (Figure 2C). Immunoblots with a monoclonal anti-human lamin A detected a protein migrating at ∼66 kDa in DT40 cells while nothing is detected using this antibody in chicken muscle tissue. On the other hand, we observed a strong expression of lamin B2 in DT40 cells compared with muscle tissue. This highly expressed lamin B2 (migrating at 66 kDa) might be recognized by our anti-human lamin A, giving this cross-reacting band. In control experiments, lamins A and C were readily detected in HeLa cells, but not in Jurkat T-lymphoma cells (Figure 2C). Lamins B1 and B2 are cleaved during apoptosis induced by etoposide both in wild-type and in caspase-6–/– cells, though not to the same extent: lamin B2 is almost completely cleaved in wild type, but is only cleaved to ∼50% in caspase-6–/– cells (Figure 2D). This last observation is consistent with the fact that caspase-6 is known to be partly involved in the cleavage of B-type lamins.
Caspase-6 is required for completion of chromatin condensation and formation of apoptotic bodies from nuclei containing lamin A
In order to examine further the role of lamin cleavage in nuclear disassembly, we turned to a cell-free system in which HeLa cell nuclei were induced to undergo apoptotic morphological changes in the presence of extracts from wild-type and caspase-6–/– DT40 cells. To perform this analysis, cytosolic extracts (Lazebnik et al., 1993) were prepared from wild-type, caspase-6+/– and caspase-6–/– DT40 cells following exposure to etoposide or staurosporine for varying lengths of time.
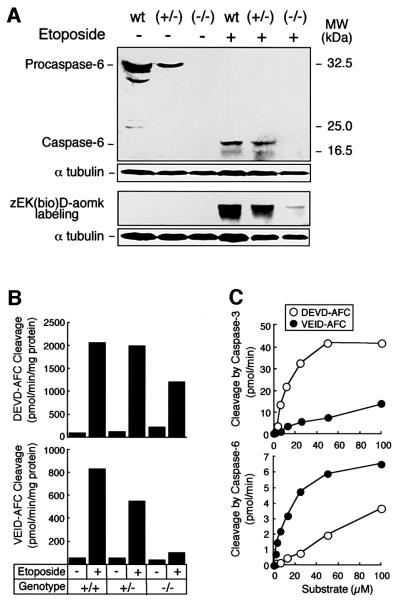
Extracts were characterized in terms of caspase-6 expression and activation, and for caspase activity. Immunoblotting analysis revealed that the caspase-6 in apoptotic extracts is processed: active caspase-6 (large subunit) appeared at 20 kDa in both the wild-type and heterozygous extracts (Figure 3A). Labelling of caspases using zEK(bio)D-aomk confirmed the strong activation of several caspase species in these extracts, with a major labelled band appearing at ∼20 kDa. Caspase-6 and caspase-3 have been shown to be the major effector caspases activated during apoptosis (Faleiro et al., 1997; Martins et al., 1997b). Interestingly, the overall level of caspase labelling with zEK(bio)D-aomk was significantly reduced in caspase-6–/– extracts relative to that seen in wild-type and caspase-6+/– extracts (Figure 3A). This could indicate either that caspase-6 is the major caspase activated in DT40 cells under these conditions, or that caspase-6 is required for the activation of other caspases in these cells.
Fig. 3. Characterization of the in vitro apoptosis system and apoptotic extracts from DT40 cells. (A) Caspase-6 expression analysis by immunoblotting in extracts from wild-type, (+/–) and (–/–) cells treated with 10 µM etoposide or diluent for 5 h. When caspase-6 in the apoptotic extracts is processed, the antibody (R549) recognizes the large subunit at ∼20 kDa. The lower panel shows the labelling of active caspases by zEK(bio)D-aomk. α-tubulin expression is used as gel loading control. (B) Measurement of DEVD-AFC and VEID-AFC cleavage activity in cytosol from etoposide-treated DT40 cells. Similar results were obtained when cytosol was prepared from staurosporine-treated DT40 clones (data not shown). (C) Evaluation of the selectivity of recom binant caspases-3 and -6 toward DEVD-AFC and VEID-AFC. Note the substantial cleavage of VEID-AFC by caspase-3 and DEVD-AFC by caspase-6 under widely used reaction conditions despite the relatively low affinity of the enzymes for these non-preferred substrates.
In an independent approach, caspase-6-like activity was assessed in these extracts by measuring VEID-AFC cleavage (Figure 3B, lower panel). This activity was substantially decreased in extracts from the caspase-6–/– cells. Although a low level of activity remained, control experiments (Figure 3C, upper panel) indicated that cleavage of VEID-AFC by caspase-3 is sufficient to account for this. Interestingly, extracts from the caspase-6–/– cells also showed a modest reduction in the ability to cleave DEVD-AFC, a substrate usually considered to be specific for caspase-3-like enzymes. Control experiments (Figure 3C, lower panel) indicated that loss of cleavage of DEVD-AFC by caspase-6 might account for this decrease in activity, although the results shown in Figure 3A raise the possibility that deletion of caspase-6 also results in a decrease in the level of caspase-3 activation in these cells.
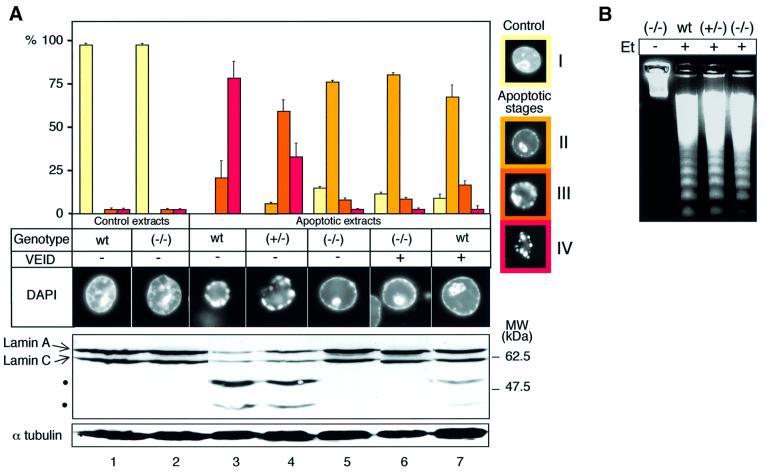
To determine whether apoptotic extracts lacking caspase-6 were able to induce apoptosis in HeLa nuclei, we defined four sequential stages (I–IV) of the chromatin condensation process (Figure 4A, right) and then scored the morphology of nuclei following exposure to extracts from etoposide-treated wild-type, heterozygote and caspase-6–/– cytosolic extracts after 2 h of incubation at 37°C (Figure 4A, left). After incubation in extracts from apoptotic wild-type cells, 22 ± 11% of HeLa nuclei displayed condensation of chromatin into discrete peripheral clumps (stage III) and 78 ± 14% had been fragmented into typical apoptotic bodies (stage IV). Similar results were observed in extracts from apoptotic caspase-6+/– cells, except that more nuclei displayed discrete clumps of peripheral chromatin and fewer had fragmented into apoptotic bodies (Figure 4A). A very different picture emerged with the caspase-6–/– extracts, revealing clear defects in chromatin condensation and formation of apoptotic bodies in HeLa nuclei (Figure 4A). In addition to a low percentage (15 ± 2%) of non-apoptotic nuclei, the great majority (75 ± 2%) of HeLa nuclei incubated in the caspase-6–/– apoptotic extracts appeared to be blocked in an early stage of chromatin condensation (stage II) after 2 h incubation and remained in this stage for up to 5 h (data not shown). Equivalent chromatin condensation defects were also observed using extracts from staurosporine-treated caspase-6–/– cells (data not shown; Figure 7A). The same effect was observed when a relatively selective inhibitor of caspase-6, z-VEID-fmk, was added to wild-type apoptotic extracts: 70% of HeLa nuclei were blocked at a similar early stage of chromatin condensation (Figure 4A). Despite these changes in chromatin condensation, this experiment failed to detect any defect in oligonucleosomal fragmentation of the DNA in caspase-6–/– extracts (Figure 4B).
Fig. 4. A block in apoptotic chromatin condensation in caspase-6-deficient apoptotic extracts. Isolated HeLa nuclei were incubated for 2 h in the apoptotic extracts previously characterized from wild-type, (+/–) and (–/–) cells (see Figure 3) in the presence (+) or absence (–) of the caspase-6-specific inhibitor VEID-fmk at 1 µM. (A) For each extract, the number of nuclei in apoptotic stages (I–IV) was determined. The stages were defined following DAPI staining of HeLa nuclei incubated in etoposide-treated DT40 apoptotic extracts or control DT40 extracts. HeLa nuclei incubated in control extracts define stage I. After addition to apoptotic extracts, the chromatin begins to condense against the nuclear periphery and nucleoli (stage II). Next, the peripheral chromatin ring condenses into discrete masses that separate from one another while the condensed chromatin from the nucleolus migrates to the nuclear periphery (stage III). Finally, the chromatin masses form discrete apoptotic bodies and the nuclear shape is lost (stage IV). A nucleus representative of the major population is shown for each stage. Data shown represent three independent experiments with an average of 300 nuclei counted per condition. After the 2 h incubation in the extracts, lamin A/C cleavage from HeLa nuclei was assessed by immunoblotting using the monoclonal antibody to human lamin A/C, JOL2. The cleavage products are shown by black dots. α-tubulin expression is used as gel loading control. Controls in this experiment include extracts from untreated wild-type cells, which gave 98% of uncondensed (non-apoptotic) nuclei upon incubation. The low (2%) frequency of apoptotic nuclei seen in controls reflects apoptotic cells in the HeLa population used to prepare nuclei, as this low percentage is always observed even at early incubation times. (B) Analysis of DNA fragmentation in the HeLa nuclei after the 2 h incubation in the extracts.
Fig. 7. Lamin A cleavage by caspase-6 is required for chromatin condensation and nuclear disassembly. (A) Isolated HeLa, Jurkat or Jurkat:GFP–lamin A nuclei were incubated for 2 h in extracts from wild-type and caspase-6–/– cells treated with 1 µM staurosporine or diluent for 8 h. A nucleus representative of the major population is shown for each condition (DAPI) along with the GFP in the Jurkat:GFP–lamin A nuclei. The signal in the panel indicated by a white asterisk was enhanced relative to that in the other panels in order to see the residual GFP–lamin A fluorescence. (B) After the 2 h incubation in the extracts, the integrity of lamin A/C and B1 from HeLa nuclei was assessed by immunoblotting using the antibody to lamin A/C (JOL2) and the antibody to lamin B1 (clone L-5). The cleavage products are indicated by black dots. Non-adjacent wells on the same blot have been juxtaposed to compose each panel in this figure. The signal in lanes 7–10 was enhanced relative to that in the other panels in order to see the GFP–lamin A band.
Since the best known substrate of caspase-6 is lamin A, we examined lamin cleavage following incubation of HeLa nuclei in the various apoptotic extracts. As expected, cleaved lamin A was detected following incubation of the nuclei with cytosolic extracts made from both wild-type and caspase-6+/– cells, but not following incubation of nuclei in the caspase-6–/– extracts (Figure 4A, lower).
These results indicate that caspase-6 is essential for the complete disassembly of HeLa cell nuclei in the in vitro apoptosis system.
Expression of exogenous caspase-6 in caspase-6–/– cells rescues the nuclear disassemblydefects in vitro
In order to confirm that the chromatin condensation and lamin cleavage defects described above were due solely to a loss of caspase-6 activity, we transfected the caspase-6–/– cells with a construct containing the chicken caspase-6 cDNA under the control of a cytomegalovirus (CMV) promoter. Clones were isolated and expression of the exogenous caspase-6 was assayed by northern blot and immunoblot analysis (Figure 5A). One of these clones (RC6) showing a level of exogenous caspase-6 expression relatively close to wild type was selected for further study. Apoptotic extracts were prepared from this clone following induction of apoptosis by etoposide and assayed for the ability to induce apoptotic morphological changes in HeLa nuclei in vitro (Figure 5B). After 2 h incubation, nuclei in RC6 extracts showed a level of chromatin condensation similar to those incubated in the wild-type extracts. These results show that the exogenous caspase-6 transfected into the caspase-6–/– clone totally reversed the nuclear disassembly defects due to caspase-6 deficiency.
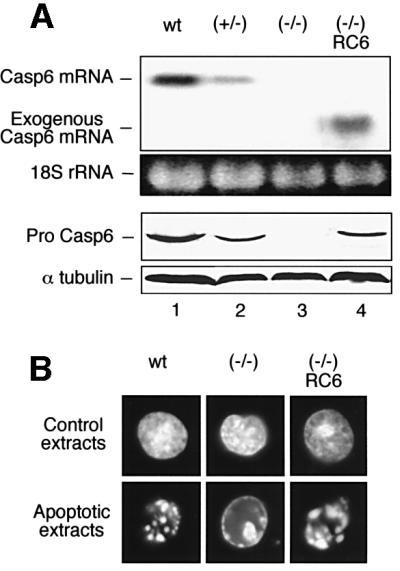
Fig. 5. The knockout phenotype is rescued by exogenous caspase-6. The caspase-6–/– cells were transfected with a construct containing the chicken caspase-6 cDNA under control of a CMV promoter. (A) Analysis of the exogenous caspase-6 expression in one transfected clone RC6. Upper panels: mRNA expression analysed by northern blotting. Lower panels: protein expression analysed by immunoblotting using polyclonal anti-caspase-6 (R549). (B) In vitro changes induced in HeLa nuclei by control and apoptotic extracts from wild-type, caspase-6–/– and caspase-6–/–/RC6 cells.
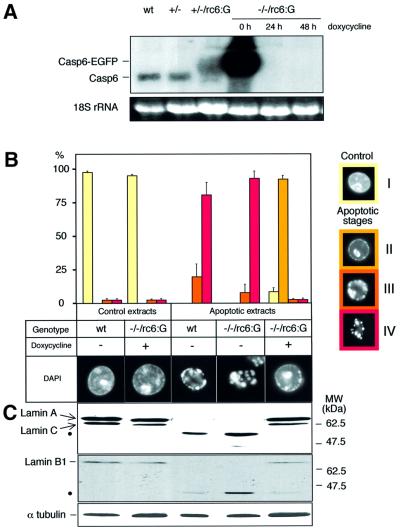
To confirm these results further, we analysed another independent knockout clone. This clone was obtained by transfecting a caspase-6+/– clone with a plasmid expressing chicken caspase-6 fused at the C-terminus to enhanced green fluorescent protein (EGFP), and then subsequently deleting the second caspase-6 allele. This caspase-6–/–/casp-6:EGFP clone (confirmed by Southern blotting, data not shown) expressed the caspase-6:EGFP fusion protein under the control of the tetracycline-repressible system (Gossen and Bujard, 1992). The expression of this transgene was rapidly repressed by the tetracycline analogue doxycycline (Figure 6A). Extracts from caspase-6–/–/casp-6:EGFP cells induced apoptosis in added HeLa nuclei normally in vitro if the cells were grown under conditions where EGFP:caspase-6 was expressed, but showed the same chromatin condensation block seen with extracts from caspase-6–/– cells if grown under conditions where the EGFP:caspase-6 was repressed (presence of doxycycline in the medium for 2 weeks) (Figure 6B). These results indicated that exogenous caspase-6 fused to EGFP is capable of rescuing the observed phenotype.
Fig. 6. The knockout phenotype is observed in an independent caspase-6-deficient clone. Analysis of an independent knockout clone (designated caspase-6–/–/casp-6:EGFP in the text and –/–/rc6:G in the figure) expressing the caspase-6:EGFP fusion protein under the control of the tetracycline-repressible system. (A) mRNA expression of endogenous caspase-6 and caspase-6:EGFP in the wild-type, +/–, +/–/rc6:G clones and –/–/rc6:G clone grown in the absence or presence of 1 µg/ml doxycycline for 24 and 48 h. (B) Isolated HeLa nuclei were incubated for 2 h in apoptotic extracts from wild-type cells and from –/–/rc6:G cells grown in the absence or presence of 1 µg/ml doxycycline for 2 weeks prior to induction of apoptosis. Apoptotic nuclei were counted according to the procedure described in Figure 4A. (C) After the 2 h incubation in the extracts, lamin A/C and B1 integrity in HeLa nuclei was assessed by immunoblotting using the antibody to lamin A/C (JOL2) and the antibody to lamin B1 (clone L-5). α-tubulin is used as gel loading control. The cleavage products are indicated by black dots.
As expected, cleaved lamin A was detected in both the wild-type and caspase-6–/–/casp-6:EGFP apoptotic extracts but not in the caspase-6–/–/casp-6:EGFP extracts following repression of the EGFP:caspase-6 (Figure 6C). Lamin B1 appeared to be totally cleaved in the wild-type and caspase-6–/–/casp-6:EGFP apoptotic extracts, but was only partially cleaved in the caspase-6–/–/casp-6:EGFP extracts following repression of the EGFP:caspase-6, suggesting that caspase-6 may collaborate with other caspases in lamin B1 cleavage. These results confirmed, using an independent knockout clone, that caspase-6 is required for lamin A cleavage and the completion of chromatin condensation in HeLa nuclei.
When lamin A is present, its cleavage is required for chromatin condensation and nuclear disassembly
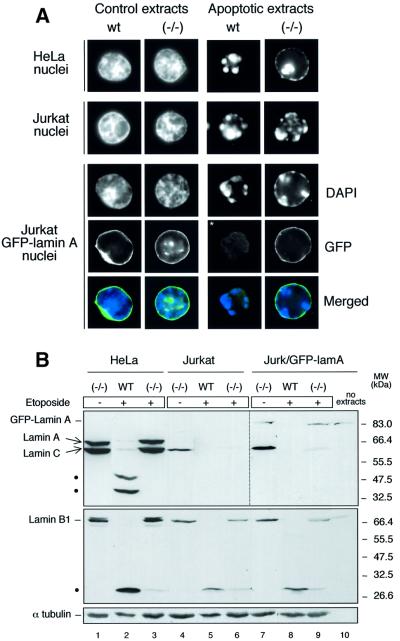
A final series of experiments was performed to determine whether the chromatin condensation defect observed in HeLa nuclei was due to the presence of uncleaved lamin A or to a requirement for caspase-6 action on some other substrate. As indicated above (Figure 2C), Jurkat T-lymphoma cells do not express detectable lamin A, and we have therefore compared the induction of apoptosis in vitro in Jurkat and HeLa cell nuclei using extracts prepared from control or staurosporine-treated wild-type and caspase-6–/– cells. As expected, after 2 h incubation in apoptotic extracts from wild-type cells, both Jurkat and HeLa nuclei showed normal condensed chromatin and formation of apoptotic bodies (Figure 7A). Strikingly, Jurkat nuclei incubated in caspase-6–/– apoptotic extracts also showed essentially normal chromatin condensation and nuclear disassembly, whilst, as before, the HeLa nuclei showed only early stage chromatin condensation. These results indicate that a critical substrate of caspase-6 whose cleavage is required for nuclear disassembly is not present in Jurkat cell nuclei, suggesting that this substrate could be lamin A.
To confirm the specific requirement for lamin A cleavage in apoptotic chromatin condensation and nuclear disassembly, Jurkat cells were stably transfected with a cDNA expressing GFP–human lamin A. This fusion protein localizes normally to the nuclear periphery, and undergoes a normal cycle of assembly and disassembly during mitosis (data not shown). Jurkat nuclei expressing GFP–lamin A were then isolated and assayed for their ability to undergo chromatin condensation and nuclear disassembly in extracts from wild-type and caspase-6–/– DT40 cells (Figure 7A). Interestingly, nuclei of Jurkat cells expressing GFP–lamin A behaved like HeLa nuclei in the extracts. They underwent normal disassembly accompanied by cleavage of GFP–lamin A in apoptotic extracts from wild-type DT40 cells, but exhibited an early block in chromatin condensation and nuclear disassembly in the caspase-6–/– extracts. Furthermore, the GFP– lamin A appeared to remain in a normal rim structure.
Figure 7B shows a biochemical analysis of this experiment. Jurkat nuclei ordinarily lack lamin A (lanes 4–6). When GFP–lamin A was present (lanes 7–10), it was cleaved only in the wild-type apoptotic extracts (lane 8) but not in the caspase-6–/– extracts (lane 9). As expected, both HeLa and Jurkat nuclei contain lamin B1. This was completely cleaved in the wild-type apoptotic extracts, but only partially cleaved in the caspase-6–/– extract. Following incubation in non-apoptotic DT40 extracts, a polypeptide co-migrating with lamin C could be detected in HeLa and Jurkat nuclei. The solubility properties of this cross-reacting protein differ from those expected for lamins, and the identity of this species remains uncertain.
Together, these results strongly suggest that lamin A is a critical substrate that, where present, must be cleaved by caspase-6 in order for nuclear apoptosis to go to completion.
Discussion
We report here the derivation of a number of chicken B-lymphoma DT40 cell lines in which the caspase-6 gene has been disrupted. Characterization of these cells indicates that caspase-6 is dispensable for the induction of apoptosis, but does appear to be required for the completion of chromatin condensation and formation of apoptotic bodies when nuclei express lamin A. These experiments yield important information about the role of both caspase-6 in apoptosis and lamin A in nuclear structure.
Caspase-6-specific functions during the apoptotic process
Although it is well known that caspase-6 can cleave lamin A adjacent to the sequence VEID (Takahashi et al., 1996a), little is known about the role(s) of caspase-6 in apoptosis in vivo. Our results reveal that in cells lacking lamin A, caspase-6 appears to be dispensable for apoptotic execution in vivo and in vitro, as judged by chromatin condensation and nuclear disassembly, production of an oligonucleosomal ladder, TUNEL labelling and annexin-V labelling (data not shown). Unlike caspase-3, which cleaves a broad range of substrates (Stroh and Schulze-Osthoff, 1998; Earnshaw et al., 1999; Slee et al., 2001), caspase-6 appears to be a more specialized enzyme. Thus, with the exception of lamin A (see below), for which there is apparently no alternative cleavage pathway, the various caspase-6 substrates must either be cleaved by other caspases or are dispensable for cell nuclei to achieve a normal apoptotic morphology in vivo and in vitro. We were surprised to note that apoptotic extracts from the caspase-6–/– cells showed a reduction in the level of DEVD-AFC cleavage activity, implying a decrease in the level of activation of caspase-3-like enzymes. This suggests that caspase-6 in DT40 cells might act upstream to activate caspase-3, as has already been shown in another system (Allsopp et al., 2000). Our results suggest that if caspase-6 does have any essential role in apoptosis in vivo, this is likely to be in terminally differentiated cells that express lamin A.
A non-apoptotic role for caspase-6?
Our initial gene targeting experiments strongly suggested that caspase-6 is an essential gene. For example, the first allele targeting of the caspase-6 gene to yield the heterozygote occurred with a frequency of 8%. When the same knockout construct (with a different selectable marker) was introduced into the heterozygotes, no homologous recombinants were obtained in 306 clones screened (all had acquired the second drug resistance marker). Curiously, when the same experiment was performed in the absence of selection for the first marker, we observed specific gene targeting in 16% of clones obtained. In this case, all recombinants had retargeted the first allele. When we then transfected the heterozygotes with cDNAs expressing caspase-6 or caspase-6:EGFP, we then obtained up to 7.5% homologous targeting into the second allele, with the percentage of recombination showing a direct correlation with the level of expression of exogenous caspase-6. Normally, these data would be taken as rigorous evidence that caspase-6 is an essential gene; however, during the screening, one of the clones that initially was expressing caspase-6 apparently lost the exogenous sequences. This clone is caspase-6 null by Southern, northern and immunoblotting, as well as caspase-6 activity assay, but grows normally, demonstrating conclusively that caspase-6 is not essential for life. In ongoing studies, we are exploring the interesting possibility that caspase-6 may be essential either for homologous recombination or for some other aspect of the growth of DT40 cells under the conditions used to obtain homologous recombinants.
Role of caspase-6 in apoptotic chromatin condensation
Previous studies have yielded conflicting results concerning the role of caspase-6 in apoptotic chromatin condensation. In one in vitro study, several factors were isolated which could promote apoptotic chromatin condensation in nuclei of permeabilized HeLa cells in the presence of active caspase-3. One of these was the novel protein acinus; another was caspase-6 (Sahara et al., 1999). A more recent study, also using an in vitro system, looked at the induction of apoptotic events in nuclei isolated from 293T cells (Slee et al., 2001). In contrast to the results presented here, that study saw no effect of caspase-6 depletion on chromatin condensation or nuclear disintegration during apoptosis. We could not find reference to the composition of the nuclear lamina in 293T cells, and it is possible that these cells do not express lamin A.
Another factor that has been shown to induce apoptotic chromatin condensation in isolated HeLa nuclei in the absence of other cytosolic factors is the apoptotic nuclease CAD/DFF40 (Samejima et al., 1998). Interestingly, the terminal phenotype of nuclei seen in CAD–/– cells closely resembles that described here when nuclei expressing lamin A undergo apoptosis in caspase-6–/– extracts (Samejima et al., 2001). We believe that this resemblance is coincidental, as DNA fragmentation occurs to a normal extent and with normal kinetics in caspase-6–/– cells. Our results differ significantly from those obtained by transfection of cultured cells with non-cleavable lamin mutants, in which the cells expressing mutant lamins showed a delay in both DNA fragmentation and chromatin condensation (Rao et al., 1996).
Uncleaved lamin A prevents chromatin condensation and nuclear disassembly during apoptotic execution
Our studies identify lamin A as a substrate of caspase-6 that apparently cannot be cleaved by other caspases during apoptosis. In addition to yielding information about one role for caspase-6, this observation also sheds light on the role of lamin A in nuclear structure.
Expression of dominant-negative mutants of lamin A can perturb DNA replication (Spann et al., 1997; Moir et al., 2000) and transcription (Lourim and Lin, 1992), and models in which lamin A has a role in organizing the chromatin at the nuclear periphery have become popular. However, evidence supporting these models comes primarily from in vitro studies and, to date, no evidence has been obtained for a role for lamin A in organizing the peripheral chromatin in intact nuclei.
Published studies have suggested two possible mechanisms by which lamin A could have a role in organizing the peripheral chromatin. A number of biochemical studies suggested that the tail and rod domains of lamins can bind directly to DNA, chromatin and core histones (Burke, 1990; Collard et al., 1990; Glass et al., 1993; Taniura et al., 1995; Rzepecki et al., 1998). More recent work has suggested that lamin A might make indirect interactions with the chromatin through a chain of interactions involving the LEM family proteins emerin and lamin-associated protein 2 (LAP2) (Lin et al., 2000). Emerin and LAP2α interact strongly with A-type lamins (Foisner and Gerace, 1993; Clements et al., 2000; Sakaki et al., 2001). Both emerin and LAP2 bind a recently discovered protein called BAF (barrier to autointegration factor) through interactions with their LEM domains (Furukawa, 1999; Haraguchi et al., 2001; Shumaker et al., 2001). BAF is a highly conserved general double-stranded DNA-binding protein that forms complexes in vitro containing multiple BAF subunits and multiple DNA molecules (Zheng et al., 2000). Thus, it has been proposed that BAF may serve as a DNA bridging factor. Elimination of BAF by RNAi in Caenorhabditis elegans gives rise to chromosome segregation defects (Zheng et al., 2000). BAF is also required for the proper targeting of lamin A, emerin and LAP2 to the nuclear envelope following mitosis (Haraguchi et al., 2001). Emerin binds to lamin A and BAF using distinct sites, and it has been proposed that a putative ternary complex of lamin A–emerin–BAF could serve to tether the chromatin at the nuclear periphery (Haraguchi et al., 2001; Lee et al., 2001).
We have shown that when lamin A is present, its cleavage is required for the completion of apoptotic chromatin condensation and apoptotic body formation. In the absence of lamin A cleavage, the chromatin collapses against the nuclear periphery, but it is unable then to fully condense further into spherical apoptotic bodies. This behaviour of the chromatin is consistent with its remaining tethered against the nuclear envelope, and suggests that one role of lamin cleavage in its rod domain during apoptosis may be to free the chromatin from the lamina. Contrasting results were obtained in a previous study in which lamin A disassembly in mitosis was inhibited by mutating key serine residues phosphorylated by Cdk1–cyclin B kinase (Heald and McKeon, 1990). In that study, mitotic chromatin condensation and segregation appeared normal despite the persistence of the transfected human lamin A in a peripheral rim-like structure. It is worth noting, however, that the mechanism of chromatin condensation in mitosis (presumably driven by phosphorylation and the action of condensin proteins) and apoptosis (apparently driven by the action of CAD nuclease; Samejima et al., 1998, 2001) differs. Furthermore, that study did not take into account the behaviour of the endogenous lamina, which could not be visualized with the antibodies used.
Lamin A cleavage appears to be essential for nuclear disassembly in apoptosis: even when lamin B1 is cleaved, the uncleaved lamin A maintains a peripheral structure that tethers the chromatin. This first evidence for lamin A-dependent tethering of the chromatin at the nuclear periphery supports recent proposals that this protein may have an important role in the establishment or maintenance of nuclear architecture.
Materials and methods
Cell culture
The chicken lymphoma B-cell line DT40 was cultured as previously described (Buerstedde and Takeda, 1991). Cell viability was assessed by morphological integrity of cells using Trypan Blue staining under phase-contrast microscopy. Chicken muscle tissue was obtained from Sainsbury’s (Edinburgh) (chicken breast, class A).
Targeted disruption of the caspase-6 gene
An 18.5 kb fragment containing the caspase-6 gene locus was isolated from a λFIX II DT40 genomic library using full-length chicken caspase-6 cDNA as a probe (isolated from a chicken DU249 cDNA λZAP library using a 400 bp cDNA sequence obtained by RT–PCR from the DU249 cell line). To construct the targeting vector, a 3 kb KpnI–XbaI fragment upstream of the start codon and a 2.1 kb XbaI–ApaI fragment downstream of the stop codon were subcloned into pBluescript. The knockout vectors were obtained by inserting a puromycin- or histidinol-resistant cassette in between the 5′ and 3′ arms. The knockout constructs (each 20 µg) were linearized with KpnI and used to electroporate 2 × 106 DT40 cells (950 µF; 300 V on a Bio-Rad Gene Pulser system). Stable transfectants were selected by limiting dilution in 0.5 µg/ml puromycin or 0.5 mg/ml histidinol for 10 days. After amplification of resistant clones, DNA was extracted and analysed on Southern blot.
Southern blot analysis
Homozygous and heterozygous clones were distinguished from the wild type by BamHI digestion of DNA. Genomic DNA (5 µg) was digested with BamHI, separated on an agarose gel and transferred to Hybond N nylon membrane (Amersham). Hybridization was performed in a phosphate buffer (1 M Na2HPO4/NaH2PO4 pH 7.4, 7% SDS) at 65°C using an external 3′ probe (ApaI–BamHI) labelled by random primer extension (Amersham).
Isolation of RNA and northern blot analysis
Total RNA was isolated according to the procedure described by Chomczynski and Sacchi (1987). A 20 µg aliquot of total RNA was separated on a 1.1% agarose/10% formaldehyde gel then blotted on a Hybond N nylon membrane (Amersham). Full-length chicken caspase-6, -7 and -3 cDNA were used as probes.
DNA fragmentation analysis
Cells or nuclei were pelleted and immediately disrupted in DNA lysis buffer (10 mM Tris–HCl pH 8, 100 mM EDTA, 10 mM EGTA, 0.5% SDS). DNase-free RNase was added to 20 µg/ml of lysate, which was incubated for 2 h at 37°C. Cell lysates were then incubated at 56°C in the presence of proteinase K at 100 µg/ml for 1 h. The DNA was extracted with phenol, pelleted with 2 vols of ethanol and 0.1 vol. of 10 M ammonium acetate, dissolved in TE-buffer (10 mM Tris–HCl pH 8, 1 mM EDTA) and separated on a 1.5% agarose gel.
Preparation of cell extracts
Cytosolic extracts were prepared from untreated and apoptotic wild-type, heterozygote and knockout DT40 cells. Cells were treated with 10 µM etoposide for 5 h or with 1 µM staurosporine for 8 h. Cells were pelleted and washed once in phosphate-buffered saline (PBS) and once in KPM buffer [50 mM PIPES pH 7.0, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 50 µM cytochalasin B, phenylmethylsulfonyl fluoride (PMSF), chymopain, leupeptin, antipain and pepstatin]. Cells were then lysed by three cycles of freeze–thaw, then sonicated and centrifuged at 190 000 g for 2 h at 4°C. The clear supernatant was collected, aliquoted and frozen at –80°C.
Preparation of HeLa nuclei and in vitro apoptosis induction
HeLa nuclei were prepared as described previously (Wood and Earnshaw, 1990). Briefly, HeLa cells were washed once in PBS and once in nuclei buffer (10 mM PIPES pH 7.4, 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 20 µM cytochalasin B and protease inhibitors). Cells were allowed to swell on ice for 20 min and then gently lysed with a Dounce homogenizer. The nuclei were layered over a 30% sucrose bed, centrifuged at 800 g for 10 min and resuspended in storage buffer (10 mM PIPES pH 7.4, 80 mM KCl, 20 mM NaCl, 250 mM sucrose, 5 mM EGTA, 1 mM DTT, 0.5 mM spermidine, 0.2 mM spermine, protease inhibitors and 50% glycerol).
For apoptosis induction, nuclei were washed twice in MDB buffer (10 mM PIPES pH 7.0, 50 mM NaCl, 5 mM EGTA, 2 mM MgCl2, 1 mM DTT) before addition of apoptotic extracts and an ATP-regenerating system (2 mM ATP, 10 mM creatine phosphate, 50 µM creatine kinase). Mixtures were incubated at 37°C for the indicated time, and 1 µl of nuclei was fixed in 2% paraformaldehyde and stained with 4′,6-diamidino- 2-phenylindole (DAPI). The remainder of each sample was lysed either in Laemmli buffer for immunoblotting analysis (see below) or in DNA lysis buffer for DNA fragmentation analysis (see above).
Immunoblotting and antibodies
Cytosolic extracts (30 µg of protein) were boiled for 5 min in Laemmli buffer containing β-mercaptoethanol, subjected to SDS–PAGE and blotted onto nitrocellulose membranes (Amersham). After transfer, proteins were visualized with Ponceau S staining (Sigma) to confirm equal protein loading. Membranes were blocked with 5% skimmed milk in PBS, then incubated with a specific antibody diluted in PBS/2% milk for 2 h at 25°C. After washing, membranes were incubated with horseradish peroxidase (HRP)-linked secondary antibody (Amersham) for 30 min at 25°C. Each of these steps was followed by three washes for 7 min in PBS/2% milk. Labelling was performed as described in the enhanced chemiluminescence (ECL) protocol (Amersham).
Monoclonal antibody (mAb) to chicken lamin B1 was purchased from Zymed Laboratories (clone L-5), mAb to human lamin A (clone JOL-2) from Abcam (Cambridge, UK) and mAb to human α-tubulin (clone B512) from Sigma. mAbs to chicken lamin A (clone 4B4-11) and to chicken lamin B2 (clone 2B8-17) were provided by Dr Reimer Stick. Rabbit polyclonal antibody to chicken caspase-6 (R549) was raised against the large subunit of caspase-6.
Caspase affinity labelling and activity analysis
Cytosolic extracts (30 µg of protein) were pre-incubated in the presence of 1 µM zEK(bio)D-aomk (Peptide Institute, Kyoto, Japan) for 15 min at 37°C before being boiled for 5 min in Laemmli buffer and processed through SDS–PAGE. After transfer and blocking in PBS/5% milk, membranes were incubated with HRP-linked streptavidin (Sigma) for 3 h at 25°C, washed and revealed by ECL.
Cleavage of DEVD-AFC (Biomol, Plymouth Meeting, PA) and VEID-AFC (Enzyme Systems Products, Dublin, CA) was assayed as previously described (Martins et al., 1997a; Kottke et al., 1999). In brief, 50 µg of cytosolic protein in 50 µl of buffer A (25 mM HEPES pH 7.5 at 4°C, 5 mM MgCl2, 1 mM EGTA, 1 mM PMSF, 10 µg/ml pepstatin A and 10 µg/ml leupeptin) or 0 µg of protein (as a blank) were diluted with 225 µl of freshly prepared buffer B [25 mM HEPES pH 7.5, 0.1% (w/v) CHAPS, 10 mM DTT, 100 U/ml aprotinin, 1 mM PMSF] containing 100 µM substrate and incubated for 2 h at 37°C. After reactions were terminated by addition of 1.225 ml of ice-cold buffer B, fluorescence was measured using an excitation wavelength of 360 nm and emission wavelength of 475 nm. Standards containing 0–15 nmol of AFC were utilized to determine the amount of fluorochrome released.
To evaluate the substrate specificity of various caspases, 10 ng of recombinant human caspase-3 or caspase-6 (PharMingen, San Diego, CA) were incubated with seven substrate concentrations (serial 2-fold dilutions starting at 100 µM) for 2 h and assayed for fluorochrome release as described above.
Acknowledgments
Acknowledgements
We thank Larry Karnitz (Mayo Research Foundation, Rochester, MN) for the gift of the GFP:lamin A plasmid, and Reimer Stick (University of Bremen) for generously providing the monoclonal antibodies to chicken lamin A and B2. We also thank Richard Adams, Xavier Fant, Ciaran Morrison and Kumiko Samejima for comments on the manuscript. This work was supported by grants from The Wellcome Trust and Neurology CEDD at GlaxoSmithKline. W.C.E. is a Principal Research Fellow of The Wellcome Trust.
References
- Allsopp T.E., McLuckie,J., Kerr,L.E., Macleod,M., Sharkey,J. and Kelly,J.S. (2000) Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ., 7, 984–993. [DOI] [PubMed] [Google Scholar]
- Benavente R., Krohne,G. and Franke,W.W. (1985) Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell, 41, 177–190. [DOI] [PubMed] [Google Scholar]
- Bonne G. et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nature Genet., 21, 285–288. [DOI] [PubMed] [Google Scholar]
- Budihardjo I., Oliver,H., Lutter,M., Luo,X. and Wang,X. (1999) Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol., 15, 269–290. [DOI] [PubMed] [Google Scholar]
- Buerstedde J.-M. and Takeda,S. (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell, 67, 179–188. [DOI] [PubMed] [Google Scholar]
- Burke B. (1990) On the cell-free association of lamins A and C with metaphase chromosomes. Exp. Cell Res., 186, 169–176. [DOI] [PubMed] [Google Scholar]
- Byun Y., Chen,F., Chang,R., Trivedi,M., Green,K.J. and Cryns,V.L. (2001) Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ., 8, 443–450. [DOI] [PubMed] [Google Scholar]
- Caulin C., Salvesen,G.S. and Oshima,R.G. (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol., 138, 1379–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P. and Sacchi,N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem., 162, 156–159. [DOI] [PubMed] [Google Scholar]
- Clements L., Manilal,S., Love,D.R. and Morris,G.E. (2000) Direct interaction between emerin and lamin A. Biochem. Biophys. Res. Commun., 267, 709–714. [DOI] [PubMed] [Google Scholar]
- Cohen G.M. (1997) Caspases: the executioners of apoptosis. Biochem. J., 326, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard J.F., Senecal,J.L. and Raymond,Y. (1990) Differential accessibility of the tail domain of nuclear lamin A in interphase and mitotic cells. Biochem. Biophys. Res. Commun., 173, 363–369. [DOI] [PubMed] [Google Scholar]
- Earnshaw W.C., Martins,L.M. and Kaufmann,S.H. (1999) Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu. Rev. Biochem., 68, 383–424. [DOI] [PubMed] [Google Scholar]
- Enari M., Sakahira,H., Yokoyama,H., Okawa,K., Iwamatsu,A. and Nagata,S. (1998) A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature, 391, 43–50. [DOI] [PubMed] [Google Scholar]
- Faleiro L., Kobayashi,R., Fearnhead,H. and Lazebnik,Y. (1997) Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J., 16, 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J.S. (2000) Pushing the envelope on lipodystrophy. Nature Genet., 24, 103–104. [DOI] [PubMed] [Google Scholar]
- Foisner R. and Gerace,L. (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes and binding is modulated by mitotic phosphorylation. Cell, 73, 1267–1279. [DOI] [PubMed] [Google Scholar]
- Franke W.W. (1987) Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell, 48, 3–4. [DOI] [PubMed] [Google Scholar]
- Furukawa K. (1999) LAP2 binding protein 1 (L2BP1/BAF) is a candidate mediator of LAP2–chromatin interaction. J. Cell Sci., 112, 2485–2492. [DOI] [PubMed] [Google Scholar]
- Galande S., Dickinson,L.A., Mian,I.S., Sikorska,M. and Kohwi-Shigematsu,T. (2001) SATB1 cleavage by caspase 6 disrupts PDZ domain-mediated dimerization, causing detachment from chromatin early in T-cell apoptosis. Mol. Cell. Biol., 21, 5591–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F.G., Thornberry,N.A., Ruffolo,S.C., Nicholson,D.W. and Roy,S. (1998) Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J. Biol. Chem., 273, 17102–17108. [DOI] [PubMed] [Google Scholar]
- Glass C.A., Glass,J.R., Taniura,H., Hasel,K.W., Blevitt,J.M. and Gerace,L. (1993) The α-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J., 12, 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M. and Bujard,H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA, 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilly M.N., Bensussan,A., Bourge,J.F., Bornens,M. and Courvalin,J.C. (1987) A human T lymphoblastic cell line lacks lamins A and C. EMBO J., 6, 3795–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T., Koujin,T., Segura-Totten,M., Lee,K.K., Matsuoka,Y., Yoneda,Y., Wilson,K.L. and Hiraoka,Y. (2001) BAF is required for emerin assembly into the reforming nuclear envelope. J. Cell Sci., 114, 4575–4585. [DOI] [PubMed] [Google Scholar]
- Heald R. and McKeon,F. (1990) Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell, 61, 579–589. [DOI] [PubMed] [Google Scholar]
- Hengartner M.O. (2000) The biochemistry of apoptosis. Nature, 407, 770–776. [DOI] [PubMed] [Google Scholar]
- Hirata H., Takahashi,A., Kobayashi,S., Yonehara,S., Sawai,H., Okazaki,T., Yamamoto,K. and Sasada,M. (1998) Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas-induced apoptosis. J. Exp. Med., 187, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H. (1989) Additional members of the rat liver lamin polypeptide family: structural and immunological characterization. J. Biol. Chem., 264, 13946–13955. [PubMed] [Google Scholar]
- Kottke T.J., Blajeski,A.L., Martins,L.M., Mesner,P.W.J., Davidson,N.E., Earnshaw,W.C., Armstrong,D.K. and Kaufmann,S.H. (1999) Comparison of paclitaxel-, 5-fluoro-2′-deoxyuridine- and epidermal growth factor (EGF)-induced apoptosis. Evidence for EGF-induced anoikis. J. Biol. Chem., 274, 15927–15936. [DOI] [PubMed] [Google Scholar]
- Kuida K., Zheng,T.S., Na,S., Kuan,C., Yang,D., Karasuyama,H., Rakic,P. and Flavell,R.A. (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature, 384, 368–372. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Cole,S., Cooke,C.A., Nelson,W.G. and Earnshaw,W.C. (1993) Nuclear events of apoptosis in vitro in cell-free mitotic extracts: a model system for analysis of the active phase of apoptosis. J. Cell Biol., 123, 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y.A., Kaufmann,S.H., Desnoyers,S., Poirier,G.G. and Earnshaw,W.C. (1994) Cleavage of poly(ADP-ribose) polymerase by a protease with properties like ICE. Nature, 371, 346–347. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Takahashi,A., Moir,R., Goldman,R., Poirier,G.G., Kaufmann,S.H. and Earnshaw,W.C. (1995) Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc. Natl Acad. Sci. USA, 92, 9042–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.K., Haraguchi,T., Lee,R.S., Koujin,T., Hiraoka,Y. and Wilson,K.L. (2001) Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci., 114, 4567–4573. [DOI] [PubMed] [Google Scholar]
- Lin F. and Worman,H.J. (1997) Expression of nuclear lamins in human tissues and cancer cell lines and transcription from the promoters of the lamin A/C and B1 genes. Exp. Cell Res., 236, 378–384. [DOI] [PubMed] [Google Scholar]
- Lin F., Blake,D.L., Callebaut,I., Skerjanc,I.S., Holmer,L., McBurney, M.W., Paulin-Levasseur,M. and Worman,H.J. (2000) MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J. Biol. Chem., 275, 4840–4847. [DOI] [PubMed] [Google Scholar]
- Liu X., Zou,H., Slaughter,C. and Wang,X. (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell, 89, 175–184. [DOI] [PubMed] [Google Scholar]
- Lourim D. and Lin,J.J. (1992) Expression of wild-type and nuclear localization-deficient human lamin A in chick myogenic cells. J. Cell Sci., 103, 863–874. [DOI] [PubMed] [Google Scholar]
- Martins L.M. et al. (1997a) Activation of multiple interleukin-1β converting enzyme homologues in cytosol and nuclei of HL-60 human leukemia cells during etoposide-induced apoptosis. J. Biol. Chem., 272, 7421–7430. [DOI] [PubMed] [Google Scholar]
- Martins L.M. et al. (1997b) Comparison of caspase activation and subcellular localization in HL-60 and K562 cells undergoing etoposide-induced apoptosis. Blood, 90, 4283–4296. [PubMed] [Google Scholar]
- Miyashita T., Nagao,K., Krajewski,S., Salvesen,G.S., Reed,J.C., Inoue,T. and Yamada,M. (1998) Investigation of glucocorticoid-induced apoptotic pathway: processing of caspase-6 but not caspase-3. Cell Death Differ., 5, 1034–1041. [DOI] [PubMed] [Google Scholar]
- Moir R.D., Spann,T.P., Herrmann,H. and Goldman,R.D. (2000) Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J. Cell Biol., 149, 1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyormoi O., Wang,Z., Doan,D., Ruiz,M., McConkey,D. and Bar-Eli,M. (2001) Transcription factor AP-2α is preferentially cleaved by caspase 6 and degraded by proteasome during tumor necrosis factor α-induced apoptosis in breast cancer cells. Mol. Cell. Biol., 21, 4856–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth K., Chinnaiyan,A.M., Garg,M., Froelich,C.J. and Dixit,V.M. (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J. Biol. Chem., 271, 16443–16446. [PubMed] [Google Scholar]
- Pellegrini L., Passer,B.J., Tabaton,M., Ganjei,J.K. and D’Adamio,L. (1999) Alternative, non-secretase processing of Alzheimer’s β-amyloid precursor protein during apoptosis by caspase-6 and -8. J. Biol. Chem., 274, 21011–21016. [DOI] [PubMed] [Google Scholar]
- Raharjo W.H., Enarson,P., Sullivan,T., Stewart,C.L. and Burke,B. (2001) Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery–Dreifuss muscular dystrophy. J. Cell Sci., 114, 4447–4457. [DOI] [PubMed] [Google Scholar]
- Rao L., Perez,D. and White,E. (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol., 135, 1441–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepecki R., Bogachev,S.S., Kokoza,E., Stuurman,N. and Fisher,P.A. (1998) In vivo association of lamins with nucleic acids in Drosophila melanogaster. J. Cell Sci., 111, 121–129. [DOI] [PubMed] [Google Scholar]
- Sahara S., Aoto,M., Eguchi,Y., Imamoto,N., Yoneda,Y. and Tsujimoto, Y. (1999) Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature, 401, 168–173. [DOI] [PubMed] [Google Scholar]
- Sakaki M., Koike,H., Takahashi,N., Sasagawa,N., Tomioka,S., Arahata, K. and Ishiura,S. (2001) Interaction between emerin and nuclear lamins. J. Biochem. (Tokyo), 129, 321–327. [DOI] [PubMed] [Google Scholar]
- Samejima K. et al. (1998) Transition from caspase-dependent to caspase-independent mechanisms at the onset of apoptotic execution. J. Cell Biol., 143, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K. et al. (1999) Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis. J. Biol. Chem., 274, 4335–4340. [DOI] [PubMed] [Google Scholar]
- Samejima K., Tone,S. and Earnshaw,W.C. (2001) CAD/DFF40 nuclease is dispensable for high molecular weight DNA cleavage and stage I chromatin condensation in apoptosis. J. Biol. Chem., 276, 45427–45432. [DOI] [PubMed] [Google Scholar]
- Shackleton S. et al. (2000) LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nature Genet., 24, 153–156. [DOI] [PubMed] [Google Scholar]
- Shumaker D.K., Lee,K.K., Tanhehco,Y.C., Craigie,R. and Wilson,K.L. (2001) LAP2 binds to BAF·DNA complexes: requirement for the LEM domain and modulation by variable regions. EMBO J., 20, 1754–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E.A., Adrain,C. and Martin,S.J. (2001) Executioner caspase-3, -6 and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem., 276, 7320–7326. [DOI] [PubMed] [Google Scholar]
- Spann T.P., Moir,R.D., Goldman,A.E., Stick,R. and Goldman,R.D. (1997) Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J. Cell Biol., 136, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula S.M. et al. (1996) The Ced3/interleukin 1β converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2α are substrates for the apoptotic mediator CPP32. J. Biol. Chem., 271, 27099–27106. [DOI] [PubMed] [Google Scholar]
- Stroh C. and Schulze-Osthoff,K. (1998) Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ., 5, 997–1000. [DOI] [PubMed] [Google Scholar]
- Takahashi A. et al. (1996a) Cleavage of lamin A by Mch2α but not CPP32: multiple ICE-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl Acad. Sci. USA, 93, 8395–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Musy,P.-Y., Martins,L.M., Poirier,G.G., Turner,P.C., Moyer,R.W. and Earnshaw,W.C. (1996b) CrmA/SPI-2 inhibition of an endogenous ICE-related protease responsible for lamin A cleav age and apoptotic nuclear fragmentation. J. Biol. Chem., 271, 32487–32490. [DOI] [PubMed] [Google Scholar]
- Taniura H., Glass,C. and Gerace,L. (1995) A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J. Cell Biol., 131, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry N.A. (1998) Caspases: key mediators of apoptosis. Chem. Biol., 5, R97–R103. [DOI] [PubMed] [Google Scholar]
- Vigouroux C., Auclair,M., Dubosclard,E., Pouchelet,M., Capeau,J., Courvalin,J.-C. and Buendia,B. (2001) Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J. Cell Sci., 114, 4459–4468. [DOI] [PubMed] [Google Scholar]
- Wellington C.L., Leavitt,B.R. and Hayden,M.R. (2000) Huntington disease: new insights on the role of huntingtin cleavage. J. Neural Transm. Suppl., 58, 1–17. [DOI] [PubMed] [Google Scholar]
- Woo M. et al. (1998) Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev., 12, 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E.R. and Earnshaw,W.C. (1990) Mitotic chromatin condensation in vitro using somatic cell extracts and nuclei with variable levels of endogenous topoisomerase II. J. Cell Biol., 111, 2839–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Ghirlando,R., Lee,M.S., Mizuuchi,K., Krause,M. and Craigie,R. (2000) Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc. Natl Acad. Sci. USA, 97, 8997–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]