Abstract
Acute stress provokes lethal cardiac arrhythmias in the hereditary long QT syndrome. Here we provide a novel molecular mechanism linking β-adrenergic signaling and altered human ether-a-go-go related gene (HERG) channel activity. Stress stimulates β-adrenergic receptors, leading to cAMP elevations that can regulate HERG K+ channels both directly and via phosphorylation by cAMP-dependent protein kinase (PKA). We show that HERG associates with 14-3-3ε to potentiate cAMP/PKA effects upon HERG. The binding of 14-3-3 occurs simultaneously at the N- and C-termini of the HERG channel. 14-3-3 accelerates and enhances HERG activation, an effect that requires PKA phosphorylation of HERG and dimerization of 14-3-3. The interaction also stabilizes the lifetime of the PKA-phosphorylated state of the channel by shielding the phosphates from cellular phosphatases. The net result is a prolongation of the effect of adrenergic stimulation upon HERG activity. Thus, 14-3-3 interactions with HERG may provide a unique mechanism for plasticity in the control of membrane excitability and cardiac rhythm.
Keywords: 14-3-3/cAMP/HERG/PKA/potassium channel
Introduction
Adaptation of the cardiac rhythm in response to varying cardiovascular demands requires dynamic, beat-to-beat regulation of membrane excitability. Ion channels establish and regulate the membrane potential and excitability of cardiac cells. Precise control of channel activity is required for regulation of heart rhythm, as evidenced by rhythm disturbances triggered by abnormal channel activity (Roden et al., 1996; Ackerman, 1998; Vincent, 1998; Chiang and Roden, 2000). The human ether-a-go-go related gene (HERG) encodes the channel pore-forming subunit that carries the rapidly activating delayed rectifier potassium current (IKr). IKr is unique in its ability to respond to and modify the rate of membrane potential repolarization at the end of each cardiac action potential, and therefore is critical for the regulation of heart rhythm. Mutations in the HERG protein or blockade of the channel by common medications result, respectively, in either hereditary or acquired long QT syndrome (LQTS), characterized by delayed cardiac repolarization, syncope, ventricular arrhythmias and sudden death (Sanguinetti et al., 1995; Roden and Balser, 1999; January et al., 2000; Mitcheson et al., 2000; Tseng, 2001). Rhythm disturbances in LQTS are often stress induced, suggesting a link between increased β-adrenergic stimulation and cardiac ion channel activity. Recent reports demonstrate that cAMP regulates HERG channels both through protein kinase (PKA)-mediated effects and by direct interaction with the protein (Thomas et al., 1999; Cui et al., 2000, 2001; Kiehn, 2000).
An increasing number of secondary subunits and accessory binding proteins are being discovered that associate with ion channels and modify their expression, trafficking and channel activity (Abbott et al., 1999; An et al., 2000; Kuryshev et al., 2000; Leonoudakis et al., 2001; Vranova et al., 2001). Examples of several ion channels residing in dynamic macromolecular complexes of kinases, phosphatases and signaling molecules have been reported (Davare et al., 2001; Marx et al., 2001). Given HERG’s essential role in governing cardiac excitability and the growing evidence for protein–protein interactions of other channels, we investigated whether additional interactions are important in the regulation of HERG.
Members of the 14-3-3 family of proteins are highly conserved and are present in all eukaryotic organisms. Seven mammalian genes encode isoforms of 14-3-3 with varying tissue expression patterns (Fu et al., 2000). The ε isoform is the most abundant species expressed in the heart. 14-3-3 proteins exist as dimers and each subunit is capable of binding specific recognition sequences of target proteins. Binding of 14-3-3 to proteins usually occurs after phosphorylation of a serine within the target sequence. Upon binding the target phosphoproteins, a variety of functional effects may occur, including activation or deactivation of enzymatic processes, subcellular targeting, structural conformation changes and cross-bridging of two proteins.
In the present study, we report and characterize the association of HERG and 14-3-3 proteins. We show that the interaction results in altered channel activity expected to result in more rapid current activation during cardiac action potentials. Moreover, we provide evidence that this functional effect is due to a combination of 14-3-3-dependent protein cross-bridging and stabilization of the phosphorylated state of HERG. These results describe a novel role for 14-3-3 in regulation of PKA-dependent effects on the HERG protein and provide initial evidence to suggest that a macromolecular complex that integrates intracellular signals with membrane excitability may dynamically regulate HERG.
Results
HERG K+ channel interacts with 14-3-3ε in the yeast two-hybrid screen
To identify proteins that interact with HERG K+ channels, we performed a yeast two-hybrid analysis of a human heart cDNA library, using the full-length cytoplasmic N-terminus (amino acids 1–375) of HERG as a bait. A total of 930 clones of the ∼1 × 108 colonies that were screened activated reporter genes, β-galactosidase and leucine prototrophy. Of 100 randomly selected positive clones examined, 75 colonies were determined to be true positives on further evaluation. Forty of the 75 interacting clones contained cDNA encoding 14-3-3ε, the most abundant of the 14-3-3 isoforms expressed in the heart (Fu et al., 2000).
14-3-3 interacts with HERG channel protein in vitro
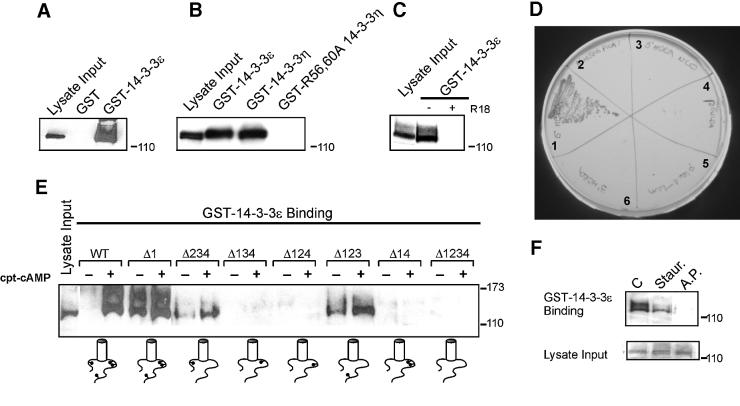
To investigate the nature of HERG interactions with 14-3-3, we used GST–14-3-3 fusion proteins for in vitro binding analyses. Wild-type HERG isolated from transfected HEK cells specifically binds to immobilized GST–14-3-3ε (Figure 1A). Both ε and η isoforms of 14-3-3 bind HERG with comparable avidity; however, the dominant-negative R56,60A mutant of 14-3-3η that dimerizes but does not bind to Raf-1 (Thorson et al., 1998) does not interact with HERG (Figure 1B). Furthermore, the interaction of HERG and 14-3-3 is abolished by the R18 peptide, a high-affinity competitive inhibitor of 14-3-3 interactions with target proteins (Wang et al., 1999) (Figure 1C).
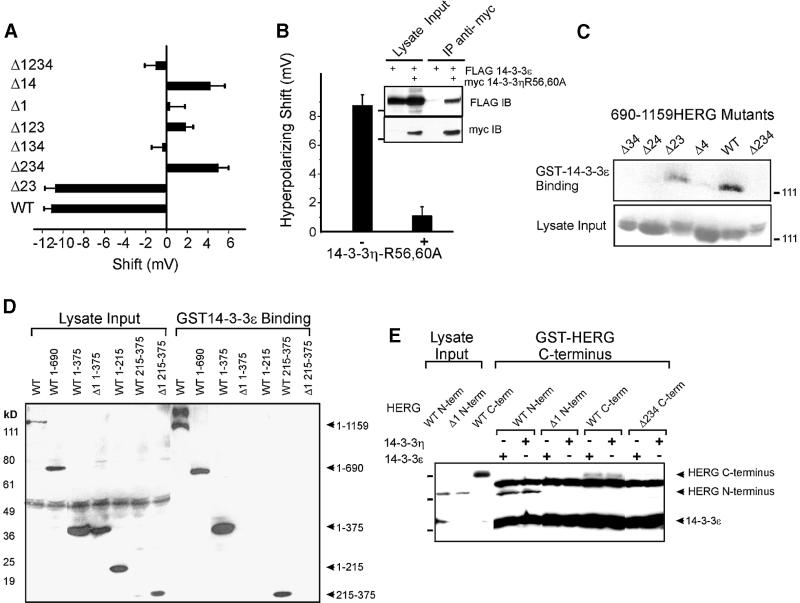
Fig. 1. Phosphorylation-dependent binding of 14-3-3 to HERG. Anti-HERG immunoblots of GST–14-3-3 binding assays. (A) Wild-type HERG (enriched for the higher molecular weight glycosylated form) binds GST–14-3-3ε but not GST. Lysate Input indicates a sample of the total cellular lysate applied to the immobilized GST fusion proteins for the binding assay. (B) HERG binds ε and η isoforms of 14-3-3 but not mutant R56,60A 14-3-3η. (C) R18 peptide blocks 14-3-3 binding to HERG. (D) Interaction of the N-terminus of HERG with 14-3-3 in yeast depends on intact S283. The EGY48 yeast strain expressing full-length 14-3-3ε was mated with the YM4271 strain expressing the pGilda plasmid containing: 1, wild-type HERG 1–375; 2, S283A HERG 1–375; 3, wild-type HERG 1–215; 4, blank; 5, pLexA-laminin; or 6, wild-type HERG 690–1159 (C-terminus). (E) Binding of wild-type and PKA site HERG mutants to GST–14-3-3ε with (+) or without (–) CPT-cAMP stimulation prior to cell lysis. Δ indicates a serine or threonine to alanine mutation of a specified PKA phosphorylation site in HERG as indicated by the schematic below. (F) Pre-treatment of HERG-expressing cells with staurosporine (Staur.) decreases 14-3-3 interaction, and alkaline phosphatase (A.P.) treatment of HERG lysates blocks 14-3-3 interaction.
14-3-3 association with HERG requires phosphorylation of HERG at S283 and S1137
Many of the identified 14-3-3-interacting proteins contain variations of the RSXpSXP recognition sequence, where phosphorylated serine (pS) is critical for binding (Muslin et al., 1996; Wang et al., 1999). Further yeast two-hybrid complementation experiments showed that the 14-3-3-binding region of HERG mapped to residues 215–375, which encompass the PKA phosphorylation site, 280RRApSSD285 (Cui et al., 2000). 14-3-3ε interacted with the full-length cytoplasmic N-terminus of HERG (residues 1–375), but failed to interact with a truncated HERG N-terminus (residues 1–215, Figure 1D). Supporting the importance of S283 in HERG’s interaction with 14-3-3 was the failure of the S283A mutant HERG bait to activate the reporter gene in yeast expressing wild-type 14-3-3ε–prey fusion protein (Figure 1D). There are a total of four PKA phosphorylation sites in HERG: S283, S890, T895 and S1137 (Thomas et al., 1999; Cui et al., 2000). To determine whether any of the remaining three PKA sites are involved in binding to 14-3-3, we performed GST–14-3-3 binding experiments with HERG bearing PKA site mutations in multiple combinations. HERG mutant Δ1 lacking S283 (S283A) and mutant Δ234 containing only S283 (S890A, T895A, S1137A) bind 14-3-3 in a cAMP-sensitive manner, suggesting that S283 is sufficient, but is not the only site for HERG–14-3-3 interaction (Figure 1E). Mutants Δ134, Δ124 and Δ14 fail to interact with 14-3-3, indicating that S890 and T895 PKA phosphorylation sites do not bind to 14-3-3 (Figure 1E). Thus, S283 and S1137 mediate 14-3-3 binding and do so independently of each other. When HERG-expressing HEK 293 cells are treated with 100 nM staurosporine, a non-specific kinase inhibitor, prior to isolation of HERG protein, we observe a decrease in HERG association with GST–14-3-3 in the binding assay (Figure 1F). Moreover, removal of phosphates from HERG by treatment with 40 U/ml alkaline phosphatase prevents HERG association with 14-3-3 (Figure 1F). These data confirm that the interaction of HERG with 14-3-3 is phosphate dependent and support the results obtained via mutagenesis of PKA phosphorylation sites. We also conclude that S283 is phosphorylated when the N-terminus of HERG is expressed in yeast but that the C-terminal S1137 may not be owing to its failure to form a functional interaction in the two-hybrid assay (Figure 1D).
14-3-3 interacts with HERG in mammalian cells
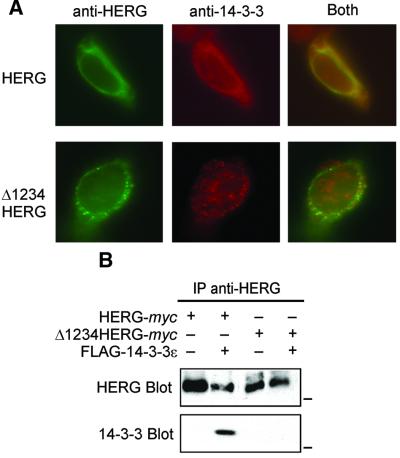
HERG–14-3-3 interaction could also be demonstrated by co-expression in HEK 293 cells. As is often seen with transient transfection of HEK cells, the heterologous expression of HERG is observed in all stages of synthesis and trafficking, with localization in the endoplasmic reticulum, Golgi and plasma membrane. Double labeling immunofluorescence shows that HERG co-localizes with a substantial portion of 14-3-3 in HEK 293 cells (Figure 2A, upper panels). When co-expressed with Δ1234 HERG, however, much less spatial overlap was observed (Figure 2A, lower panels). To confirm a physical interaction within mammalian cells, we performed co- immunoprecipitations of HERG and 14-3-3 which showed that wild-type HERG but not Δ1234 HERG associated with 14-3-3 (Figure 2B).
Fig. 2. Interaction between HERG and 14-3-3 in eukaryotic cells. (A) Double-labeled immunofluorescence of HEK 293 cells co-transfected with wild-type (upper panels) or Δ1234 HERG (lower panels) stained for 14-3-3 (red, middle panels), or HERG (green, left panels), with merged channels (right panels) demonstrating co-localization of the wild-type, but not Δ1234 HERG with 14-3-3. (B) Immunoblot analysis of HERG interaction with 14-3-3 in eukaryotic cells. HEK 293 cells were transfected with either HERG-myc or Δ1234 HERG-myc, with or without FLAG-14-3-3ε.
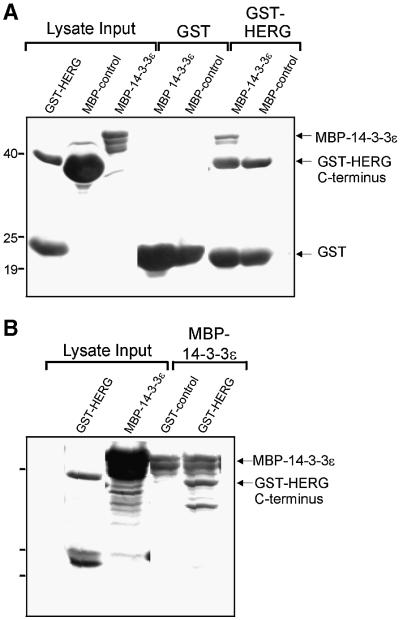
Although co-precipitation argues for an interaction between 14-3-3 and HERG protein, there is the possibility that the two are bridged by an intervening protein that exists in mammalian cells, as is seen with the Slob protein mediating the interaction between the Ca2+-activated K+ channel, dSlo, and 14-3-3 (Zhou et al., 1999). To examine the possibility that additional molecules from eukaryotic cells might mediate HERG–14-3-3 interaction, we performed in vitro binding experiments with HERG and 14-3-3ε expressed in Escherichia coli (Figure 3). We expressed GST–HERG C-terminus (residues 979–1159), purified the recombinant protein and in vitro phosphorylated it with the catalytic subunit of PKA. The immobilized GST–HERG bound maltose-binding protein (MBP)– 14-3-3 (Figure 3A) and, conversely, immobilized MBP–14-3-3 bound GST–HERG (Figure 3B). This result indicates that the physical association of HERG with 14-3-3 is direct and that no eukaryotic bridging molecules are required for the binding of the two proteins.
Fig. 3. Direct interaction of HERG and 14-3-3ε. Gels are Coomassie Blue stained for total protein. Recombinant HERG C-terminus (residues 979–1159, GST fusion) and 14-3-3ε (MBP fusion) were expressed separately in E.coli and examined for in vitro binding. (A) Purified and eluted MBP fusion proteins (MBP alone or MBP–14-3-3ε) applied to immobilized GST or GST–HERG C-terminus (after pre-phosphorylating with PKA). Expression of fusion proteins is confirmed in the purified bacterial lysates (Lysate Input). MBP–14-3-3 but not MBP alone co-precipitated with GST–HERG. GST alone failed to precipitate MBP–14-3-3. (B) Conversely, when purified GST–HERG (that had been in vitro PKA phosphorylated) was applied to immobilized MBP fusion proteins, it only co-precipitated with immobilized MBP–14-3-3ε but not with MBP alone.
14-3-3 modulates voltage-dependent activation of HERG potassium channel
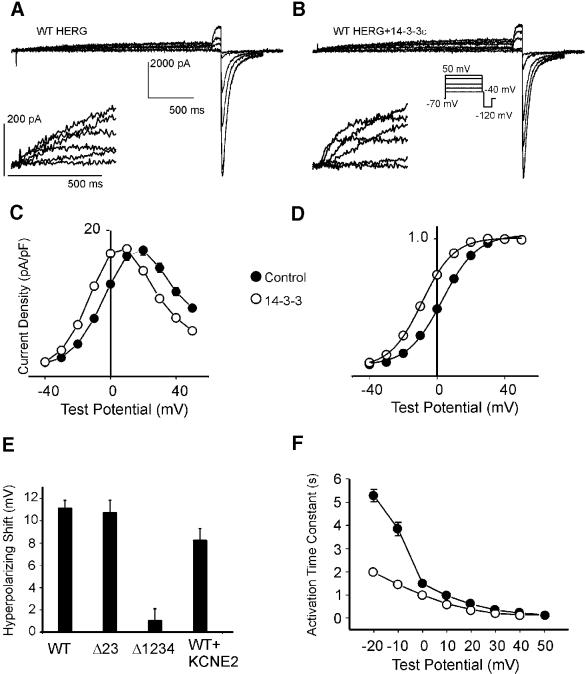
Electrophysiological K+ channel properties differed in Chinese hamster embryo (CHO) cells transfected with HERG alone compared with cells co-transfected with HERG and 14-3-3ε (Figure 4A and B). Overexpression of 14-3-3ε shifts the current–voltage (I–V) and voltage dependence of activation in a hyperpolarizing direction (Figure 4C and D). The resulting shift in voltage at half-maximal activation is –11.12 ± 0.71 mV (Figure 4E). Δ23 HERG is affected to a comparable extent by 14-3-3 overexpression (shift of –10.7 ± 0.71 mV); however, 14-3-3 fails to alter the voltage-dependent activation of Δ1234 HERG significantly (Figure 4E). In previous work from our and other laboratories (Thomas et al., 1999; Cui et al., 2000; Kiehn, 2000), cAMP produced a PKA-dependent reduction (∼40%) in HERG K+ current density, consistent with the present finding that 14-3-3 overexpression reduces the current density by ∼35%. In the heart, HERG may be associated with the accessory protein MiRP1, encoded by the KCNE2 gene (Abbott et al., 1999). Currents produced by HERG co-expressed with KCNE2 were also shifted in a hyperpolarizing direction (–8.2 ± 1.05 mV) when 14-3-3 was overexpressed (Figure 4E). The rate of development of outward current during depolarization is accelerated during 14-3-3 overexpression (Figure 4F). This acceleration is most prominent at voltages that a myocyte would experience during the repolarizing phase of a cardiac action potential. No significant alterations of reversal potential, steady-state inactivation or kinetics of deactivation are seen with 14-3-3 overexpression (for HERG versus HERG + 14-3-3ε: Ereversal = 73.4 ± 0.3 versus 75.5 ± 0.2 mV). The rate of onset of inactivation was slightly slower when 14-3-3e was overexpressed with HERG (2.2 ± 1.6 versus 2.9 ± 1.92 ms at 0 mV, for HERG and HERG + 14-3-3, respectively). 14-3-3 reduces the maximal tail current density at –40 mV (from 43.1 ± 1.9 to 28.7 ± 0.5 pA/pF, P = 0.1 for HERG and HERG + 14-3-3, respectively); however, the peak tail currents at –120 mV were unaltered (from 90.7 ± 2.0 to 91.2 ± 1.5 pA/pF). Steady-state outward current density is not significantly affected (from 17.2 ± 0.8 and 17.3 ± 0.4 pA/pF for HERG and HERG + 14-3-3, respectively). This apparent discrepancy in steady-state versus outward tail current amplitudes can be explained by the combination of a hyperpolarizing shift in the voltage dependence of activation without a concomitant shift in the voltage dependence of inactivation. Thus, 14-3-3 controls the voltage dependence of activation of IKr via the same PKA sites in HERG as those that mediate binding to 14-3-3.
Fig. 4. Functional consequences of HERG interaction with 14-3-3. Representative whole-cell current tracings from CHO cells expressing wild-type (WT) HERG alone (A) or co-expressing wild-type HERG and 14-3-3ε (WT+14-3-3ε) (B). Time and current scale bars apply to both (A) and (B). Insets show expanded views of the early development of outward current. (C) I–V curves for wild-type HERG with (open circles) or without (closed circles) overexpressed 14-3-3ε. (D) Normalized voltage-dependent activation curves with (open circles) or without (closed circles) 14-3-3ε overexpression. (E) Summary of shifts in Vh of wild-type HERG (n = 31), Δ23 HERG (n = 10), Δ1234 HERG (n = 20) and wild-type HERG + KCNE2 (n = 7) in the presence of 14-3-3ε. Vh measurements were obtained from the Boltzman fits of normalized voltage-dependent activation curves. Shifts in voltage dependence of activation were determined as the difference between Vh with 14-3-3 and Vh without 14-3-3 overexpression. (F) Time constants of onset of outward current shown for HERG with and without overexpressed 14-3-3ε.
HERG sites S283 and S1137 are both required for functional effects of 14-3-3
We further examined the individual functional contribution of each HERG–14-3-3 binding site in electrophysiological studies. Both S283 and S1137 PKA sites are required for the full 14-3-3-dependent hyperpolarizing shift in voltage dependence of activation (Figure 5A). When only one site is available (Δ123, Δ1 or Δ234), there is no hyperpolarizing shift nor is there quantitative summation of the effects of 14-3-3 on single site mutants to equal that observed when both sites 1 and 4 are intact (Figure 5A). These findings indicate that the functional effects are due to binding of 14-3-3 at both sites on HERG simultaneously.
Fig. 5. Functional effects of HERG association with 14-3-3 require dimerization of 14-3-3 and cross-bridging of HERG. (A) Summary of Vh shifts from CHO cells expressing different PKA mutants of HERG in the presence and absence of 14-3-3ε overexpression. Shifts are calculated as the difference in Vh between cells without and with 14-3-3. (B) Vh from CHO cells expressing HERG and 14-3-3ε with (+, n = 14) or without (–, n = 17) R56,60A 14-3-3η. Inset, heterodimerization of 14-3-3ε (FLAG) and R56,60A 14-3-3η (myc) confirming the mutant η isoform’s dominant-negative effect over the ε isoform (Lysate Input indicates a sample of the total cellular lysate applied to the gel without immunoprecipitation). (C and D) Anti-Myc immunoblots demonstrating the interaction of 14-3-3 with isolated C- (C) and N- (D) termini of HERG in GST–14-3-3 binding assays requiring intact PKA phosphorylation sites. (E) Anti-Myc immunoblot of simultaneous binding of HERG N- and C-termini to GST–HERG C-terminus mediated by 14-3-3(ε or η). A non-specific Myc-immunoreactive band was observed between the N- and C-terminal fragments of HERG whenever GST–HERG C-terminus-expressing bacterial lysates were probed. (Lysate Input indicates a sample of the total cellular lysate applied to the immobilized GST fusion proteins for the binding assay.)
14-3-3 must form dimers for functional regulation of HERG channels
When wild-type 14-3-3ε and dominant mutant R56,60A 14-3-3η were co-expressed in CHO cells, the hyperpolarizing shift in voltage dependence of HERG was abolished (Figure 5B). Under these conditions, wild-type 14-3-3ε can complex with mutant R56,60A 14-3-3η, forming heterodimers (Figure 5B, inset) in which one half can bind to HERG but the other cannot. Thus, the functional 14-3-3-mediated regulation of HERG channels requires that both binding sites within HERG are intact and that 14-3-3 be capable of forming cross-bridging dimers.
We next investigated whether 14-3-3 dimers can cross-link the cytoplasmic N- and C-termini of HERG by simultaneously interacting with both termini of HERG. Fragments of HERG from these regions bind to GST– 14-3-3 (Figure 5C and D). An immobilized GST–HERG C-terminal fusion protein was used to bind recombinant Myc-14-3-3 and either the Myc-tagged N- or C-terminus of HERG (Figure 5E). Under these conditions, 14-3-3 (ε or η isoform) is capable of physically cross-bridging the C-terminus of HERG to either the N- or C-terminus of HERG only when the corresponding PKA consensus site is present and phosphorylated. Therefore, direct cross-bridging of the N- and C-termini of HERG via a 14-3-3 dimer is sterically permitted.
14-3-3 increases the extent of PKA-dependent phosphorylation of HERG
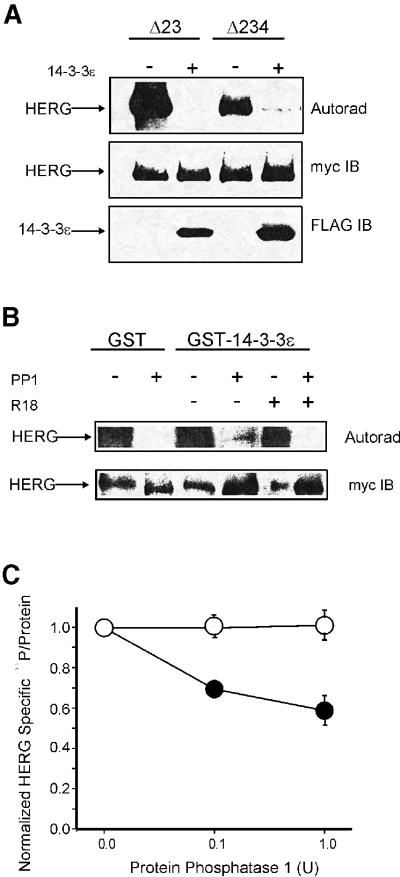
14-3-3 has been hypothesized to protect the phosphates at the binding sites of interacting proteins (Chiang et al., 2001). We examined whether 14-3-3 plays such a functional role in the turnover of phosphates on HERG. In vitro 32P phosphorylation of HERG with PKA is greatly reduced (∼90%) when 14-3-3ε is co-expressed in HEK 293 cells (Figure 6A). Such a reduction in back-phosphorylation indicates fewer phosphate-accepting groups available on HERG and is strong evidence for an increase in the endogenous PKA-phosphorylated state of the protein (Bartel et al., 1993; Chen et al., 1995; Hell et al., 1995). As the balance of protein kinase and protein phosphatase activities determines the state of phosphorylation of HERG, our finding suggests that 14-3-3 either protects the phosphates on S283 and S1137 from the action of cellular phosphatases, directly suppresses phosphatases or promotes PKA activity. To date, there has been no evidence that 14-3-3 binds to either regulatory or catalytic subunits of PKA nor that it alters the activity of the kinase, and few relevant phosphatases have been shown to be directly altered by 14-3-3 (Fu et al., 2000); accordingly, we focused our attention on 14-3-3’s effects on the stability of PKA phosphates on HERG.
Fig. 6. 14-3-3-mediated stabilization of PKA-dependent phosphates on HERG. (A) Overexpression of 14-3-3 with HERG reduces the capacity for in vitro back-phosphorylation with the catalytic subunit of PKA. Top panel: autoradiography signal from immunoprecipitated HERG (either Δ23 or Δ234 PKA mutants) phosphorylated with PKA and [32P]ATP. Middle panel: anti-Myc detection of total HERG protein from the same gel. Bottom panel: 14-3-3 co-immunoprecipitated with HERG. (B) Recombinant 14-3-3 partially shields the removal of in vitro PKA-mediated 32P labeling of HERG by 10 U of protein phosphatase 1-α (PP1) in vitro. Top panel: autoradiography signal from immunoprecipitated HERG phosphorylated with PKA and [32P]ATP. Bottom panel: anti-Myc detection of total HERG protein. R18 was added at 1 µM. (C) PP1-mediated dephosphorylation of intracellularly 32P- labeled HERG with (open circles) or without (closed circles) overexpressed 14-3-3. The specific activity of [32P]HERG was obtained as the ratio of the densitometry signal from autoradiography and anti-HERG immunoblot. Data were normalized to display relative changes with time.
We next examined the effect of 14-3-3 overexpression on the protein phosphatase 1 (PP1)-dependent dephosphorylation of HERG. Dephosphorylation of HERG by PP1 is diminished when recombinant 14-3-3 is pre-incubated with HERG that had been 32P-labeled, in vitro, with PKA (Figure 6B). This protection is abolished in the presence of a 14-3-3 inhibitory peptide, R18. Similarly, 14-3-3 overexpression with HERG helps to shield cAMP-mediated 32P-labeled HERG from subsequent application of protein phosphatase (Figure 6C). A phosphate protection mechanism that increases the basal phosphorylation state of HERG is supported further by our finding that CPT-cAMP failed to alter significantly the voltage dependence of activation or the current density from HERG when 14-3-3ε was co-expressed (basal Vh = –8.37 ± 0.58 mV, CPT-cAMP-stimulated Vh = –9.52 ± 0.12 mV, n = 10).
Discussion
Our results show that 14-3-3 dynamically associates with HERG in a phosphorylation-dependent manner at specific N- and C-terminal PKA sites. This physical association stabilizes the PKA-phosphorylated state of the channel and encourages channel opening at more negative potentials. In a single tetrameric HERG channel, there are eight potential 14-3-3-binding sites. Productive interaction with HERG requires 14-3-3 dimers simultaneously and directly to bind each of the two mapped sites on HERG, possibly forming cytoplasmic cross-bridges within and between HERG subunits. In the only other known 14-3-3 association with an animal K+ channel, an intermediate protein, Slob, is required for association of 14-3-3 with the Drosophila Slopoke (dSlo) K+ channel. In this case, 14-3-3 association significantly decreases the amplitude of the outward K+ current, with a right shift in voltage-dependent activation (Zhou et al., 1999).
Extensive structural and functional studies of 14-3-3 have identified both dimerization and target protein-binding domains (Xiao et al., 1995; Dubois et al., 1997; Ichimura et al., 1997; Yaffe et al., 1997; Rittinger et al., 1999; Obsil et al., 2001). Structural requirements for 14-3-3–target protein interactions are also known, and several consensus binding motifs within target proteins have been described, the majority of which are phosphoserine- or phosphothreonine-based. Many cellular proteins can bind 14-3-3, and members of the 14-3-3 family of proteins have been implicated in a variety of cellular functions, including signal transduction, cell cycle control and apoptosis (Clark et al., 1997; Thorson et al., 1998; Prezeau et al., 1999; Widen et al., 2000; Yip-Schneider et al., 2000; Lehoux et al., 2001). Functional characteristics of several ion channels are modified by 14-3-3 proteins (Booij et al., 1999; Zhou et al., 1999; Chan et al., 2000).
14-3-3 is capable of simultaneously binding two separate regions of a protein (Yaffe et al., 1997). Increased affinity of interaction is seen when a 14-3-3 dimer binds two consensus sites on a peptide. 14-3-3 binding to multiple sites within Raf-1 has been demonstrated to regulate Raf-1 kinase activity. In the inactive state, two of the three 14-3-3-binding sites on Raf-1 are occupied. Upon activation, 14-3-3 is displaced from the first two sites and binding of 14-3-3 to the third site stabilizes the active conformation of the kinase (Morrison and Cutler, 1997; Fu et al., 2000).
Our GST–14-3-3 binding data reveal an enrichment of the higher molecular weight HERG band (Figure 1E) that represents the mature glycosylated membrane form of the protein (Zhou et al., 1998). Endogenous PKA may phosphorylate the channel preferentially after it has trafficked to the plasma membrane. PKA is targeted to the plasma membrane via membrane-specific A-kinase anchoring proteins (AKAPs) (Rubin, 1994; Fraser and Scott, 1999), and AKAPs have been shown to enhance PKA regulation of another LQTS-related channel, KvLQT1 (Potet et al., 2001; Marx et al., 2002). The relative abundance of free 14-3-3 near the plasma membrane may therefore alter the probability of the HERG channel remaining phosphorylated. Competition by other cellular targets, in turn, may determine local concentrations of free 14-3-3 available for HERG binding (Tzivion et al., 2001). Thus, 14-3-3, by dynamically associating with HERG, may provide a mechanism for plasticity in cardiac electrophysiological response to stress.
The modification of cAMP-dependent effects on the HERG/IKr by 14-3-3 has physiological significance in that the ∼11 mV hyperpolarizing shift in voltage-dependent activation caused by the association is expected to produce a 15–45% increase in HERG/IKr activity (Figure 4D) over the voltage range (–20 to +10 mV) where the current must influence the cardiac action potential the most. An increase in IKr of this magnitude will accelerate the repolarizing phase of the action potential, shortening its duration. Thus, the reduction of IKr amplitude generally seen on PKA-dependent phosphorylation (Thomas et al., 1999; Cui et al., 2000) will be counterbalanced by earlier and faster current activation if 14-3-3 binds.
We hypothesize that HERG/Ikr exists in a macromolecular signaling complex that includes 14-3-3 and possibly AKAPs. The complex, by coordinating multiple pathways of modification of channel activity, dynamically regulates membrane excitability. Although our description and characterization of the interaction between 14-3-3 and HERG in heterologous expression systems supports an important role in IKr regulation, we do not yet know to what extent such an interaction occurs in the intact heart. It will also be important to determine how this interaction impacts triggering of arrhythmias in LQTS patients. The 14-3-3ε gene is located on chromosome 17 (17p13.3), not near to any known arrhythmogenic genetic loci (Priori et al., 1999). Patients with either hereditary or acquired LQTS are at increased risk for fatal cardiac arrhythmias that are often triggered by acute cardiovascular demands such as stress. In such states, there is augmented β-adrenergic activity and ensuing elevations in cellular cAMP. Therefore, mediation of increased PKA-dependent phosphorylation of HERG by 14-3-3 has important implications for the function of the channel and regulation of the cardiac action potential.
Materials and methods
Yeast two-hybrid screen
The two-hybrid screen was carried out using a manufacturer-suggested protocol for the Matchmaker LexA 2-Hybrid System (Clontech). Bait-transformed yeast (histidine selection) were tested negative for self-activation of β-galactosidase and leucine (Leu) reporter genes, and subsequently were transformed with an amplified human heart Matchmaker LexA cDNA library (Clontech), containing a tryptophan (Trp) selection marker. Clones carrying both the bait and the library vectors were amplified on glucose-based SD medium without Ura, Trp and His; colony numbers were titered, and ∼1 × 108 clones were screened for activation of Leu and β-galactosidase reporter genes on the induction medium (galactose/raffinose-based SD without Trp, Ura, His and Leu) with 80 mg/l X-Gal (Gibco). Clones that activated both reporter genes after 72 h of incubation at 30°C were grown in liquid medium lacking Trp and Ura in an attempt to kick out the bait vector. Yeast colonies expressing only the prey and pO8-LacZ vectors were tested for self-activation of the β-galactosidase activity. Clones that failed to activate β-galactosidase were mated with YM4271 yeast carrying either the bait or control vectors. Diploid colonies were tested for Leu and β-galactosidase activity. Plasmid DNA was isolated from true positive clones, and library inserts were amplified by PCR using pB42AD vector-specific primers. Restriction digest patterns with AluI were compared for obtained fragments from all true positive clones, and unique clones were subjected to sequencing.
Plasmids and mutagenesis
14-3-3ε cDNA was subcloned into pCDNA3 (Invitrogen), pCMV-tag3B (Stratagene), pMAL-C2 (New England Biolabs) and p3XFLAG-CMV-10 (Sigma), and pGEX-KG vectors. 14-3-3η and R56,60A 14-3-3η in PCDNA3.1 were a generous gift from Dr Andrey Shaw (Washington University, St Louis, MO). GST–14-3-3η and GST–R56,60A 14-3-3η fusion plasmids were created in the pGEX-KG vector. Preparations of the GST–HERG C-terminal (979–1159) fusion and Δ1, Δ123, Δ14, Δ234, Δ23 and Δ1234 mutants of HERG have been described previously (Cui et al., 2000, 2001). Δ2 and Δ3 mutants of HERG were constructed using a PCR-based mutagenesis system (QuikChange; Stratagene) with the following primers: Δ2-forward, 5′-GCGCAAGTTGGCCTTCCGCAGGCGCACGG-3′; Δ2-reverse, 5′-CCGTGCGCCTGCGGAAGGCCAACTTGCGC-3′; Δ3-forward, 5′ GTCCTTCCGCAGGCGCGCGGACAAGGACACG-3′; Δ3-reverse, 5′-CGTGTCCTTGTCCGCGCGCCTGCGGAAGGAC-3′.
Cell culture and transfection
CHO and HEK 293 cell lines (ATCC) were cultured under standard conditions in Ham’s F12 or RPMI 1640 (Cellgro) media supplemented with 10% fetal calf serum (BioWhittaker) and penicillin/streptomycin (Cellgro). For biochemical analyses, transient transfections were performed with Lipofectamine2000 (Life Technologies) and for electrophysiological studies transfection was done by electroporation as described previously (Kagan et al., 2000; Cui et al., 2001). Cells were treated with 500 µM CPT-cAMP and 200 µM 3-isobutyl-1-methyl xanthine (IBMX) when indicated.
Electrophysiology
Whole-cell voltage clamp studies were performed by the patch–clamp technique (Hammill et al., 1981) as described previously (Kagan et al., 2000). The extracellular solution was 150 mM NaCl, 1.8 mM CaCl2, 4 mM KCl, 1 mM MgCl2, 5 mM glucose and 10 mM HEPES buffer pH 7.4 at room temperature. The intracellular pipet solution was 126 mM KCl, 4 mM Mg-ATP, 2 mM Mg2SO4, 5 mM EGTA, 0.5 mM CaCl2 and 25 mM HEPES buffer pH 7.2 at room temperature. To elicit HERG K+ currents, depolarizing voltage pulses were applied to various levels from a holding potential of –70 mV for 3.5 s followed by partial repolarization to –40 mV and then to –120 mV briefly to measure outward and inward tail currents. Current densities were calculated as current (pA) divided by cell capacitance (pF). Voltage activation data were plotted as peak tail current amplitudes, normalized to the maximal value, against the test potential values and were fitted to a Boltzman function, I = 1/(1 + exp[(Vh – V)/k]), where I is the measured tail current, V is the applied membrane voltage, Vh is the voltage at half-maximal activation and k is the slope factor.
Immunoblot analysis and immunoprecipitation
Cells were harvested for analysis as described previously (Kagan et al., 2000; Cui et al., 2001). Briefly, 36–48 h after transfection, HEK 293 cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold NDET buffer [150 mM NaCl, 25 mM Tris–HCl pH 7.5, 5 mM EDTA, 1% NP-40, 0.4% deoxycholic acid and EDTA-free protease inhibitor cocktail tablets (Roche Pharmaceuticals)]. Lysates were cleared by centrifugation at 13 200 r.p.m. for 10 min at 4°C. For co-immunoprecipitation studies, transiently transfected cells were harvested as above, and cell lysates were incubated with 30 µl of rabbit polyclonal anti-HERG antibody generated against the N-terminus (residues 1–130) of HERG (Cui et al., 2001) or 10 µl of rabbit polyclonal A14 anti-Myc antibody (Santa Cruz) for 4 h at 4°C. Protein–antibody complexes were precipitated with 30 µl of protein A–agarose (Pierce) for an additional 3 h. Precipitated proteins were eluted with SDS–PAGE sample buffer, separated on a 4–20% gradient SDS–polyacrylamide gel and subjected to immunoblot analysis.
Binding assays
GST and MBP fusion proteins were expressed in E.coli BL21 strain. For studies using GST fusion proteins, bacteria were lysed by sonication and bound to glutathione–agarose. For MBP fusion protein purification, amylose–agarose was used to bind recombinant protein from lysed bacteria. When fusion proteins were used in solution, they were eluted with either reduced glutathione (for GST fusion proteins) or maltose (for MBP fusion proteins). HEK 293 cells expressing either wild-type or mutant HERG cDNA were harvested as described previously (McDonald et al., 1997; Kagan et al., 2000). Cell lysates were incubated with 10 µg of fusion protein immobilized on agarose beads for 4–12 h at 4°C with rotation. For 14-3-3 inhibitory peptide studies, 1 mM stock solution of R18 peptide (PHCVPRDLSWLDLEANMCL; Laboratory for Molecular Analysis, AECOM) in 10 mM Tris pH 7.5 was used. After three washes with NDET buffer, complexed proteins were eluted with SDS–PAGE sample buffer and subjected to immunoblot analysis.
In vitro phosphorylation and dephosphorylation of HERG
Samples for in vitro phosphorylation were obtained either from immunoprecipitation from HEK 293 cells overexpressing wild-type or mutant HERG with or without 14-3-3ε or from the glutathione-purified C-terminal fragment of HERG. In vitro phosphorylation was performed with purified PKA catalytic subunit (Sigma) in buffer containing 50 mM Tris–HCl pH 7.0, 10 mM MgCl2, 0.1 mg/ml bovine serum albumin (BSA), 10 µM [γ-32P]ATP or 10 µM Mg-ATP for 1 h at 30°C (Cui et al., 2000). The reaction was terminated with ice-cold NDET buffer. In vitro dephosphorylation was performed in 50 mM Tris–HCl pH 7.5, 1 mM dithiothreitol (DTT), 10% glycerol, 2 mM MnCl2, 2 mM MgCl2, 0.05% Triton X-100 with specified amounts of PP1-α (Sigma) at 30°C for 1 h.
Intracellular 32P labeling
Transfected HEK 293 cells were starved with phosphate-free Dulbecco’s modified Eagle’s medium (DMEM; Gibco) for 1 h at 37°C. Cells were labeled with 100 µCi/ml 32P (carrier-free, inorganic phosphate) in phosphate-free DMEM for 4 h at 37°C. At 15 min prior to harvesting, 500 µM CPT-cAMP and 200 µM IBMX were added to the labeling media. Cell lysates were collected and cleared as above and immunoprecipitated with 10 µl of polyclonal anti-Myc antibody (A14).
Immunofluorescence
HEK 293 cells were transiently transfected with wild-type or mutant HERG cDNA and, 24 h after transfection, cells were re-plated onto sterile glass coverslips within 35 mm tissue culture dishes. At 48 h after transfection, culture media were removed and cells were fixed with 4% paraformaldehyde for 20 min. After washing with PBS, cells were permeabilized with 0.3% Triton X-100 in PBS for 10 min, washed and blocked with 5% BSA in PBS for 30 min. Cells were incubated with primary antibodies (rabbit anti-HERG, goat anti-pan-14-3-3) in PBS and 0.1% NP-40 in PBS, and incubated in the dark with the secondary antibodies [donkey anti-goat AlexaFluor 568 (Molecular Probes) and goat anti-rabbit AlexaFluor 488 (Molecular Probes)] at 1:500 dilution for 1 h. For double labeling experiments, secondary antibodies were applied separately, each for 1 h, with donkey anti-goat preceding goat anti-rabbit, and washing between antibody applications. Samples were washed with PBS and 0.1% NP-40 in PBS as above before mounting onto the slides for examination using digital microscopy. Images were acquired using IPLab software and analyzed in Adobe Photoshop.
Acknowledgments
Acknowledgements
We thank Dr Charles Rubin for helpful discussions and suggestions, Drs Kami Kim and Glenn Fishman for critical reading of the manuscript, Dr Jie Cui for construction of several HERG-PKA mutants, Michael Rajala for MBP reagents, and Dr Andrey Shaw (Washington University, St Louis, MO) for advice and 14-3-3η cDNAs. This work was supported by a grant from the NIH/NHLBI (RO1 HS57388 to T.V.M.).
References
- Abbott G.W., Sesti,F., Splawski,I., Buck,M.E., Lehmann,M.H., Timothy, K.W., Keating,M.T. and Goldstein,S.A. (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell, 97, 175–187. [DOI] [PubMed] [Google Scholar]
- Ackerman M.J. (1998) The long QT syndrome: ion channel diseases of the heart. Mayo Clin. Proc., 73, 250–269. [DOI] [PubMed] [Google Scholar]
- An W.F. et al. (2000) Modulation of A-type potassium channels by a family of calcium sensors. Nature, 403, 553–556. [DOI] [PubMed] [Google Scholar]
- Bartel S., Karczewski,P. and Krause,E.G. (1993) Protein phosphorylation and cardiac function: cholinergic–adrenergic interaction. Cardiovasc. Res., 27, 1948–1953. [DOI] [PubMed] [Google Scholar]
- Booij P.P., Roberts,M.R., Vogelzang,S.A., Kraayenhof,R. and De Boer, A.H. (1999) 14-3-3 proteins double the number of outward-rectifying K+ channels available for activation in tomato cells. Plant J., 20, 673–683. [DOI] [PubMed] [Google Scholar]
- Chan H.C. et al. (2000) Modulation of the Ca2+-activated Cl– channel by 14-3-3ε. Biochem. Biophys. Res. Commun., 270, 581–587. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Law,B., Kondratyuk,T. and Rossie,S. (1995) Identification of soluble protein phosphatases that dephosphorylate voltage-sensitive sodium channels in rat brain. J. Biol. Chem., 270, 7750–7756. [DOI] [PubMed] [Google Scholar]
- Chiang C.E. and Roden,D.M. (2000) The long QT syndromes: genetic basis and clinical implications. J. Am. Coll. Cardiol., 36, 1–12. [DOI] [PubMed] [Google Scholar]
- Chiang C.W., Harris,G., Ellig,C., Masters,S.C., Subramanian,R., Shenolikar,S., Wadzinski,B.E. and Yang,E. (2001) Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin-3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood, 97, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Clark G.J., Drugan,J.K., Rossman,K.L., Carpenter,J.W., Rogers-Graham, K., Fu,H., Der,C.J. and Campbell,S.L. (1997) 14-3-3ζ negatively regulates raf-1 activity by interactions with the Raf-1 cysteine-rich domain. J. Biol. Chem., 272, 20990–20993. [DOI] [PubMed] [Google Scholar]
- Cui J., Melman,Y., Palma,E., Fishman,G.I. and McDonald,T.V. (2000) Cyclic AMP regulates the HERG K+ channel by dual pathways. Curr. Biol., 10, 671–674. [DOI] [PubMed] [Google Scholar]
- Cui J., Kagan,A., Qin,D., Mathew,J., Melman,Y.F. and McDonald,T.V. (2001) Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J. Biol. Chem., 276, 17244–17251. [DOI] [PubMed] [Google Scholar]
- Davare M.A., Avdonin,V., Hall,D.D., Peden,E.M., Burette,A., Weinberg,R.J., Horne,M.C., Hoshi,T. and Hell,J.W. (2001) A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science, 293, 98–101. [DOI] [PubMed] [Google Scholar]
- Dubois T. et al. (1997) Structure and sites of phosphorylation of 14-3-3 protein: role in coordinating signal transduction pathways. J. Protein Chem., 16, 513–522. [DOI] [PubMed] [Google Scholar]
- Fraser I.D. and Scott,J.D. (1999) Modulation of ion channels: a ‘current’ view of AKAPs. Neuron, 23, 423–426. [DOI] [PubMed] [Google Scholar]
- Fu H., Subramanian,R.R. and Masters,S.C. (2000) 14-3-3 proteins: structure, function and regulation. Annu. Rev. Pharmacol. Toxicol., 40, 617–647. [DOI] [PubMed] [Google Scholar]
- Hammill O.P., Marty,A., Neher,E., Sakmann,B. and Sigworth,F.J. (1981) Improved patch clamp techniques for high-resolution current recordings from cells and cell-free membrane patches. Pflügers Arch., 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hell J.W., Yokoyama,C.T., Breeze,L.J., Chavkin,C. and Catterall,W.A. (1995) Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J., 14, 3036–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T., Ito,M., Itagaki,C., Takahashi,M., Horigome,T., Omata,S., Ohno,S. and Isobe,T. (1997) The 14-3-3 protein binds its target proteins with a common site located towards the C-terminus. FEBS Lett., 413, 273–276. [DOI] [PubMed] [Google Scholar]
- January C.T., Gong,Q. and Zhou,Z. (2000) Long QT syndrome: cellular basis and arrhythmia mechanism in LQT2. J. Cardiovasc. Electrophysiol., 11, 1413–1418. [DOI] [PubMed] [Google Scholar]
- Kagan A., Yu,Z., Fishman,G.I. and McDonald,T.V. (2000) The dominant negative LQT2 mutation A561V reduces wild-type HERG expression. J. Biol. Chem., 275, 11241–11248. [DOI] [PubMed] [Google Scholar]
- Kiehn J. (2000) Regulation of the cardiac repolarizing HERG potassium channel by protein kinase A. Trends Cardiovasc. Med., 10, 205–209. [DOI] [PubMed] [Google Scholar]
- Kuryshev Y.A., Gudz,T.I., Brown,A.M. and Wible,B.A. (2000) KChAP as a chaperone for specific K+ channels. Am. J. Physiol. Cell Physiol., 278, C931–C941. [DOI] [PubMed] [Google Scholar]
- Lehoux S., Abe,J., Florian,J.A. and Berk,B.C. (2001) 14-3-3 binding to Na+/H+ exchanger isoform-1 is associated with serum-dependent activation of Na+/H+ exchange. J. Biol. Chem., 276, 15794–15800. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D., Mailliard,W., Wingerd,K., Clegg,D. and Vandenberg, C. (2001) Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J. Cell Sci., 114, 987–998. [DOI] [PubMed] [Google Scholar]
- Marx S.O., Reiken,S., Hisamatsu,Y., Gaburjakova,M., Gaburjakova,J., Yang,Y.M., Rosemblit,N. and Marks,A.R. (2001) Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J. Cell Biol., 153, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx S.O., Kurokawa,J., Reiken,S., Motoike,H., D’Armiento,J., Marks, A.R. and Kass,R.S. (2002) Requirement of a macromolecular signaling complex for β adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science, 295, 496–499. [DOI] [PubMed] [Google Scholar]
- McDonald T.V., Yu,Z., Ming,Z., Palma,E., Meyers,M.B., Wang,K.W., Goldstein,S.A. and Fishman,G.I. (1997) A minK–HERG complex regulates the cardiac potassium current IKr. Nature, 388, 289–292. [DOI] [PubMed] [Google Scholar]
- Mitcheson J.S., Chen,J., Lin,M., Culberson,C. and Sanguinetti,M.C. (2000) A structural basis for drug-induced long QT syndrome. Proc. Natl Acad. Sci. USA, 97, 12329–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D.K. and Cutler,R.E. (1997) The complexity of Raf-1 regulation. Curr. Opin. Cell Biol., 9, 174–179. [DOI] [PubMed] [Google Scholar]
- Muslin A.J., Tanner,J.W., Allen,P.M. and Shaw,A.S. (1996) Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell, 84, 889–897. [DOI] [PubMed] [Google Scholar]
- Obsil T., Ghirlando,R., Klein,D.C., Ganguly,S. and Dyda,F. (2001) Crystal structure of the 14-3-3ζ:serotonin N-acetyltransferase complex. a role for scaffolding in enzyme regulation. Cell, 105, 257–267. [DOI] [PubMed] [Google Scholar]
- Potet F., Scott,J.D., Mohammad-Panah,R., Escande,D. and Baro,I. (2001) AKAP proteins anchor cAMP-dependent protein kinase to KvLQT1/IsK channel complex. Am. J. Physiol. Heart Circ. Physiol., 280, H2038–H2045. [DOI] [PubMed] [Google Scholar]
- Prezeau L., Richman,J.G., Edwards,S.W. and Limbird,L.E. (1999) The ζ isoform of 14-3-3 proteins interacts with the third intracellular loop of different α2-adrenergic receptor subtypes. J. Biol. Chem., 274, 13462–13469. [DOI] [PubMed] [Google Scholar]
- Priori S.G. et al. (1999) Genetic and molecular basis of cardiac arrhythmias: impact on clinical management parts I and II. Circulation, 99, 518–528. [DOI] [PubMed] [Google Scholar]
- Rittinger K., Budman,J., Xu,J., Volinia,S., Cantley,L.C., Smerdon,S.J., Gamblin,S.J. and Yaffe,M.B. (1999) Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol. Cell, 4, 153–166. [DOI] [PubMed] [Google Scholar]
- Roden D.M. and Balser,J.R. (1999) A plethora of mechanisms in the HERG-related long QT syndrome. Genetics meets electrophysiology. Cardiovasc. Res., 44, 242–246. [DOI] [PubMed] [Google Scholar]
- Roden D.M., Lazzara,R., Rosen,M., Schwartz,P.J., Towbin,J. and Vincent,G.M. (1996) Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps and future directions. The SADS Foundation Task Force on LQTS. Circulation, 94, 1996–2012. [DOI] [PubMed] [Google Scholar]
- Rubin C.S. (1994) A kinase anchor proteins and the intracellular targeting of signals carried by cyclic AMP. Biochim. Biophys. Acta, 1224, 467–479. [PubMed] [Google Scholar]
- Sanguinetti M.C., Jiang,C., Curran,M.E. and Keating,M.T. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell, 81, 299–307. [DOI] [PubMed] [Google Scholar]
- Thomas D., Zhang,W., Karle,C.A., Kathofer,S., Schols,W., Kubler,W. and Kiehn,J. (1999) Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J. Biol. Chem., 274, 27457–27462. [DOI] [PubMed] [Google Scholar]
- Thorson J.A., Yu,L.W., Hsu,A.L., Shih,N.Y., Graves,P.R., Tanner,J.W., Allen,P.M., Piwnica-Worms,H. and Shaw,A.S. (1998) 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol. Cell. Biol., 18, 5229–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng G.N. (2001) IKr: the hERG channel. J. Mol. Cell. Cardiol., 33, 835–849. [DOI] [PubMed] [Google Scholar]
- Tzivion G., Shen,Y.H. and Zhu,J. (2001) 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene, 20, 6331–6338. [DOI] [PubMed] [Google Scholar]
- Vincent G.M. (1998) The molecular genetics of the long QT syndrome: genes causing fainting and sudden death. Annu. Rev. Med., 49, 263–274. [DOI] [PubMed] [Google Scholar]
- Vranova E., Tahtiharju,S., Sriprang,R., Willekens,H., Heino,P., Palva,E.T., Inze,D. and Van Camp,W. (2001) The AKT3 potassium channel protein interacts with the AtPP2CA protein phosphatase 2C. J. Exp. Bot., 52, 181–182. [PubMed] [Google Scholar]
- Wang B., Yang,H., Liu,Y.C., Jelinek,T., Zhang,L., Ruoslahti,E. and Fu,H. (1999) Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry, 38, 12499–12504. [DOI] [PubMed] [Google Scholar]
- Widen C., Zilliacus,J., Gustafsson,J.A. and Wikstrom,A.C. (2000) Glucocorticoid receptor interaction with 14-3-3 and Raf-1, a proposed mechanism for cross-talk of two signal transduction pathways. J. Biol. Chem., 275, 39296–39301. [DOI] [PubMed] [Google Scholar]
- Xiao B., Smerdon,S.J., Jones,D.H., Dodson,G.G., Soneji,Y., Aitken,A. and Gamblin,S.J. (1995) Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature, 376, 188–191. [DOI] [PubMed] [Google Scholar]
- Yaffe M.B., Rittinger,K., Volinia,S., Caron,P.R., Aitken,A., Leffers,H., Gamblin,S.J., Smerdon,S.J. and Cantley,L.C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell, 91, 961–971. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider M.T., Miao,W., Lin,A., Barnard,D.S., Tzivion,G. and Marshall,M.S. (2000) Regulation of the Raf-1 kinase domain by phosphorylation and 14-3-3 association. Biochem. J., 351, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Gong,Q., Epstein,M.L. and January,C.T. (1998) HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J. Biol. Chem., 273, 21061–21066. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Schopperle,W.M., Murrey,H., Jaramillo,A., Dagan,D., Griffith,L.C. and Levitan,I.B. (1999) A dynamically regulated 14-3-3, Slob, and Slowpoke potassium channel complex in Drosophila presynaptic nerve terminals. Neuron, 22, 809–818. [DOI] [PubMed] [Google Scholar]