Abstract
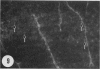
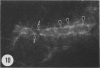
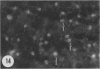
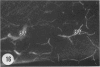
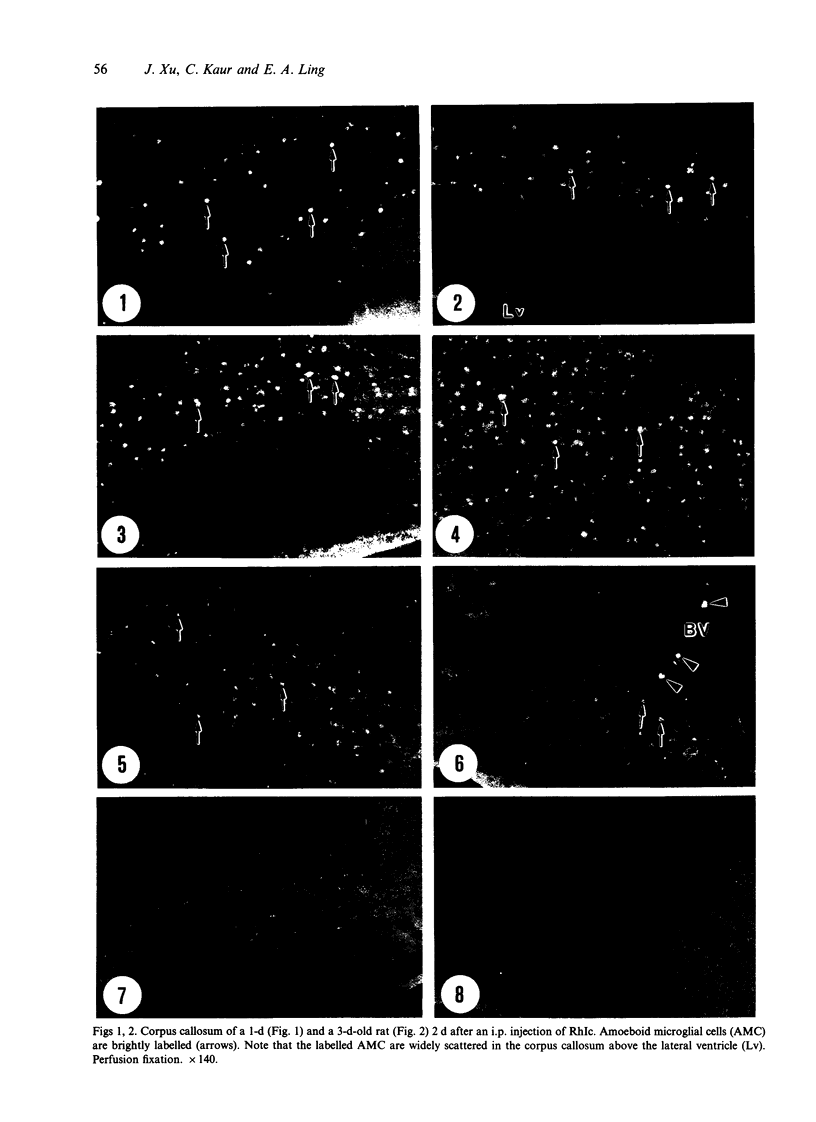
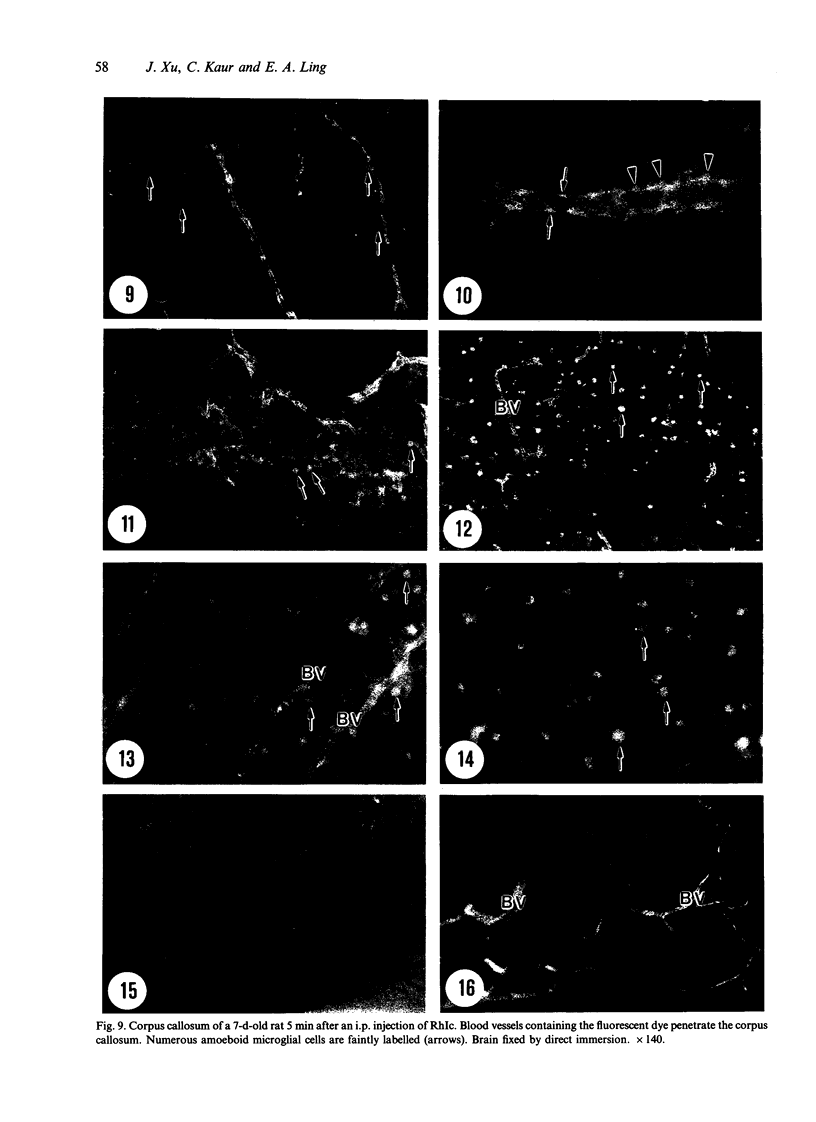
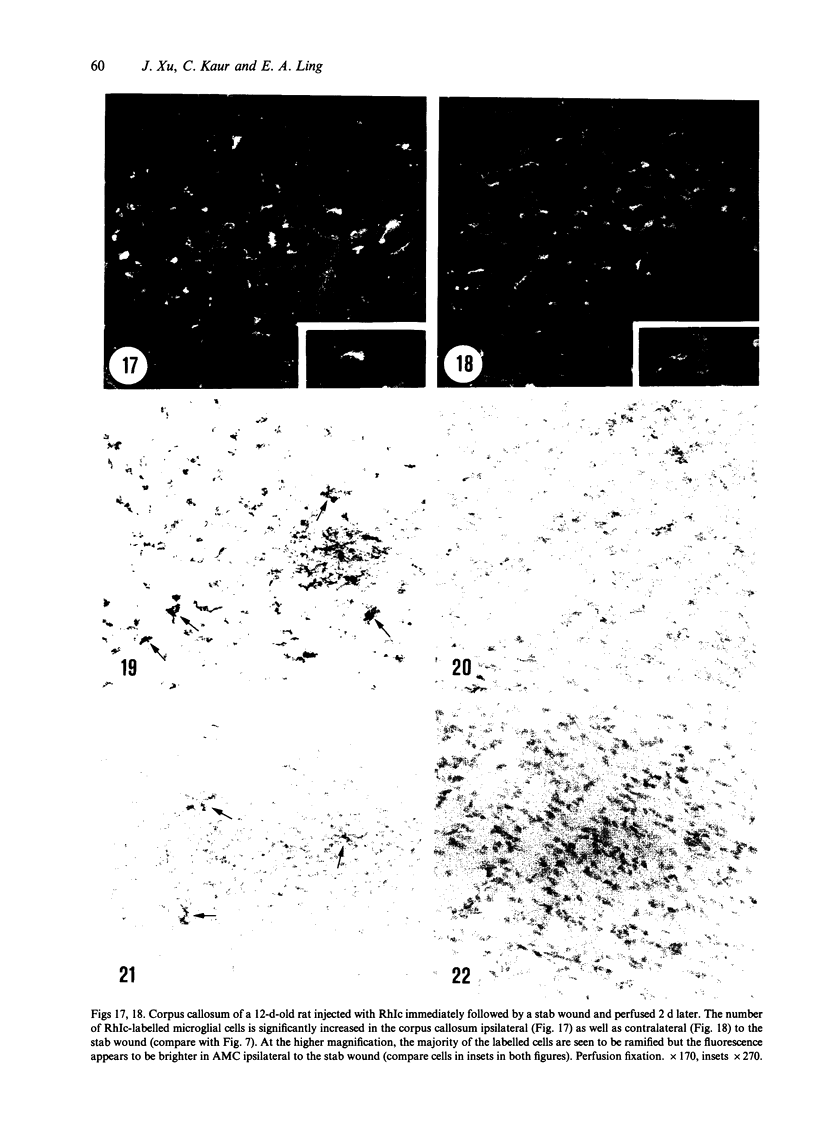
Amoeboid microglial cells (AMC) in the corpus callosum were selectively labelled following a single intraperitoneal (i.p.) injection of the fluorescent dye, rhodamine isothiocyanate (RhIc) into postnatal rats. The frequency of RhIc-labelled cells varied with age, with the largest number occurring in 7-d-old animals. Thereafter, the labelled cells declined drastically in number and fluorescence and were barely detectable in 12-d-old injected rats. Labelled cells were absent in 13-d or older rats given an RhIc injection. When the injected RhIc was followed over a time course sequence, it was first detected in the cerebral blood vessels and their lining endothelia within 5 min after the injection. A variable number of AMC emitting a weaker fluorescence were closely adherent to the outer walls of the blood vessels. With time, the fluorescence in the AMC was progressively enhanced, but that in the blood vessels showed a concomitant reduction. In the rats that received an intravenous (i.v.) injection of RhIc, the labelling pattern of AMC, both in terms of its variation with age and in temporal sequence, paralleled that in rats given i.p. injections. In 12-d-old rats subjected to a stab wound coupled with an i.p. injection of RhIc, a considerable number of AMC not normally labelled at this age were activated. The cells exhibited an intense fluorescence and expressed MHC surface antigen immunoreactivity. It is concluded from this study that when injected i.p. or i.v., RhIc is readily circulated to the cerebral vessels, where it enters brain tissue by transendothelial transport.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cancilla P. A., Baker R. N., Pollock P. S., Frommes S. P. The reaction of pericytes of the central nervous system to exogenous protein. Lab Invest. 1972 Apr;26(4):376–383. [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Scanning electron microscopy of transitory subependymal cysts in the developing midbrain of postnatal rats. Arch Histol Cytol. 1989 Jul;52(3):311–317. doi: 10.1679/aohc.52.311. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Leong S. K., Ling E. A. Amoeboid and ramified microglia: their interrelationship and response to brain injury. Glia. 1992;6(1):39–47. doi: 10.1002/glia.440060106. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Expression of major histocompatibility complex and leukocyte common antigens in amoeboid microglia in postnatal rats. J Anat. 1991 Aug;177:117–126. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Kaur L. C., Yick T. Y., Wong W. C. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anat Embryol (Berl) 1990;182(5):481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Mato M., Ookawara S., Kurihara K. Uptake of exogenous substances and marked infoldings of the fluorescent granular pericyte in cerebral fine vessels. Am J Anat. 1980 Mar;157(3):329–332. doi: 10.1002/aja.1001570308. [DOI] [PubMed] [Google Scholar]
- Mato M., Ookawara S., Mato T. K., Namiki T. An attempt to differentiate further between microglia and fluorescent granular perithelial (FGP) cells by their capacity to incorporate exogenous protein. Am J Anat. 1985 Feb;172(2):125–140. doi: 10.1002/aja.1001720203. [DOI] [PubMed] [Google Scholar]
- Mato M., Ookawara S., Sugamata M., Aikawa E. Evidence for the possible function of the fluorescent granular perithelial cells in brain as scavengers of high-molecular-weight waste products. Experientia. 1984 Apr 15;40(4):399–402. doi: 10.1007/BF01952574. [DOI] [PubMed] [Google Scholar]
- Mato M., Ookawara S., Tooyama K., Ishizaki T. Chronobiological studies on the blood-brain barrier. Experientia. 1981;37(9):1013–1015. doi: 10.1007/BF01971811. [DOI] [PubMed] [Google Scholar]
- McRae A., Ling E. A., Polinsky R., Gottfries C. G., Dahlström A. Antibodies in the cerebrospinal fluid of some Alzheimer's disease patients recognize amoeboid microglial cells in the developing rat central nervous system. Neuroscience. 1991;41(2-3):739–752. doi: 10.1016/0306-4522(91)90364-t. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Fluorescent granular perithelial cells and granular pial cells in the brains of aged mice. J Hirnforsch. 1987;28(4):435–443. [PubMed] [Google Scholar]
- Tseng C. Y., Ling E. A., Wong W. C. Scanning electron microscopy of amoeboid microglial cells in the transient cavum septum pellucidum in pre- and postnatal rats. J Anat. 1983 Mar;136(Pt 2):251–263. [PMC free article] [PubMed] [Google Scholar]
- Vorbrodt A. W., Lossinsky A. S., Wisniewski H. M. Localization of alkaline phosphatase activity in endothelia of developing and mature mouse blood-brain barrier. Dev Neurosci. 1986;8(1):1–13. doi: 10.1159/000112236. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Dontenwill M., Bricca G. Postnatal development of the endothelial system of the rat cerebellum using immunohistochemical techniques. Brain Res. 1985 Jan;349(1-2):253–257. doi: 10.1016/0165-3806(85)90149-x. [DOI] [PubMed] [Google Scholar]