Abstract
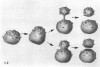
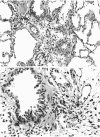
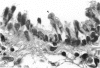
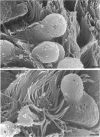
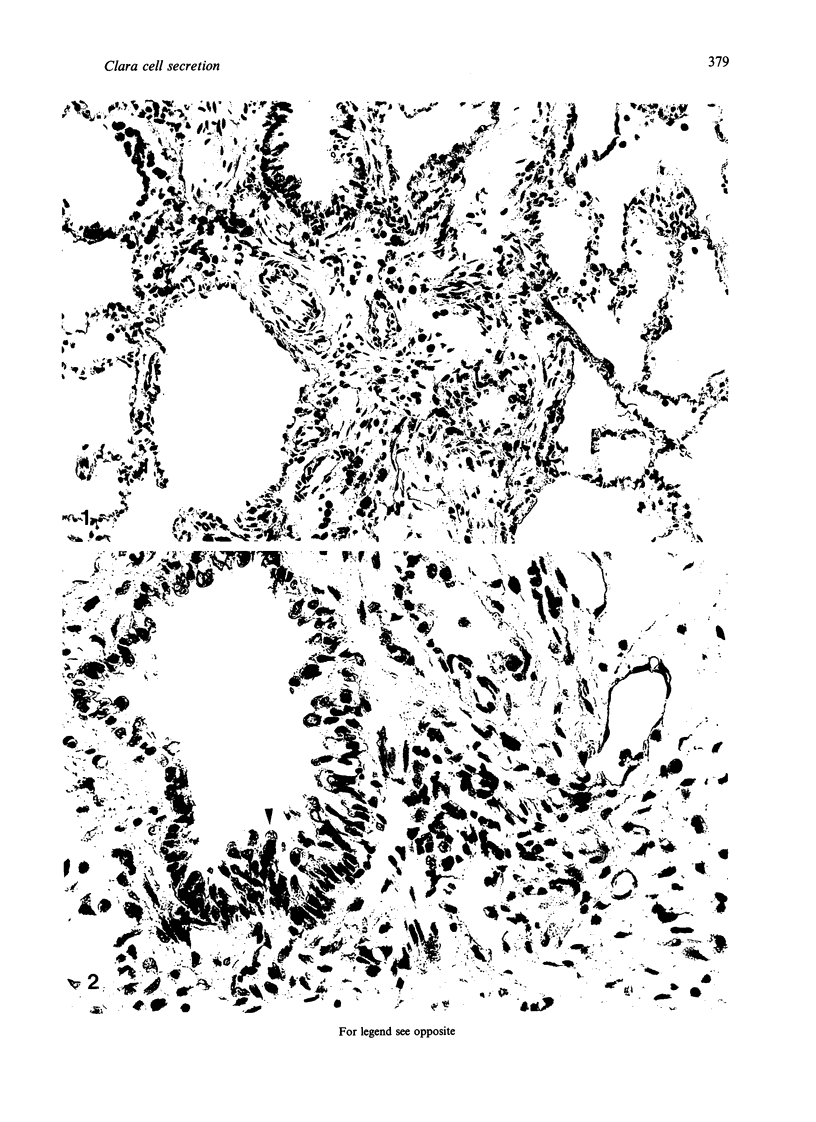
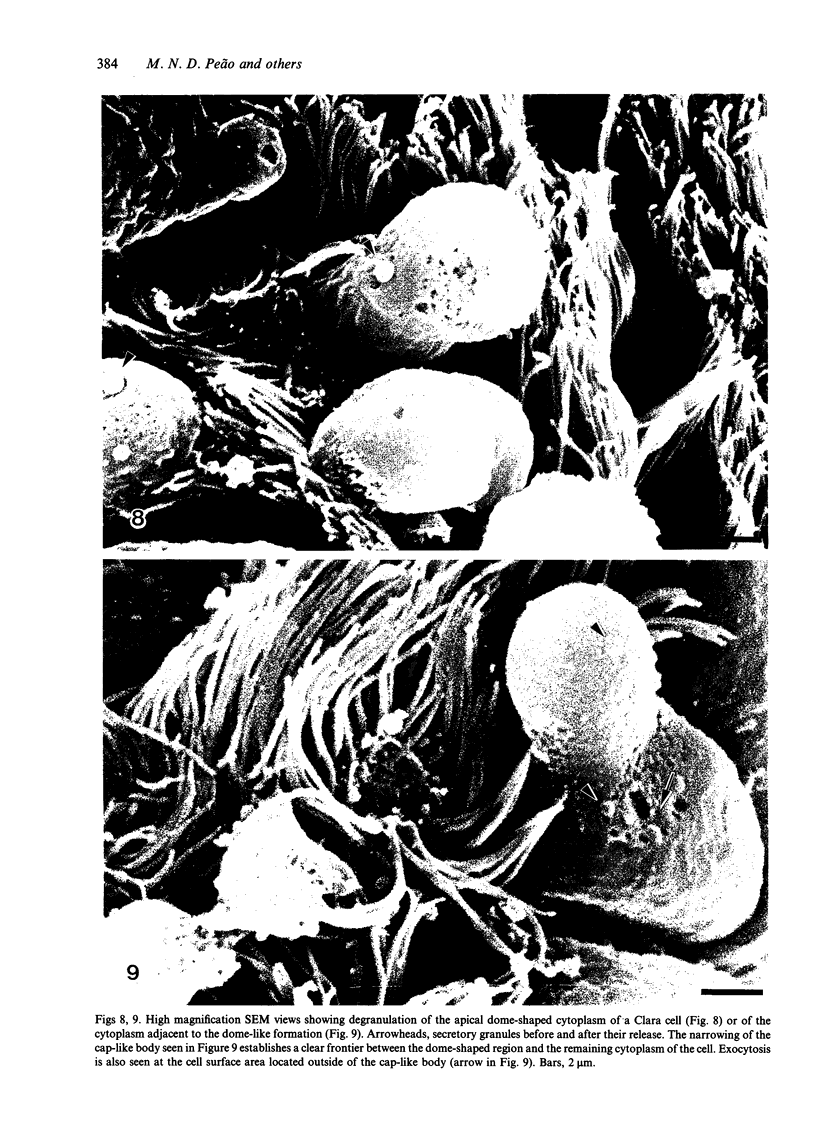
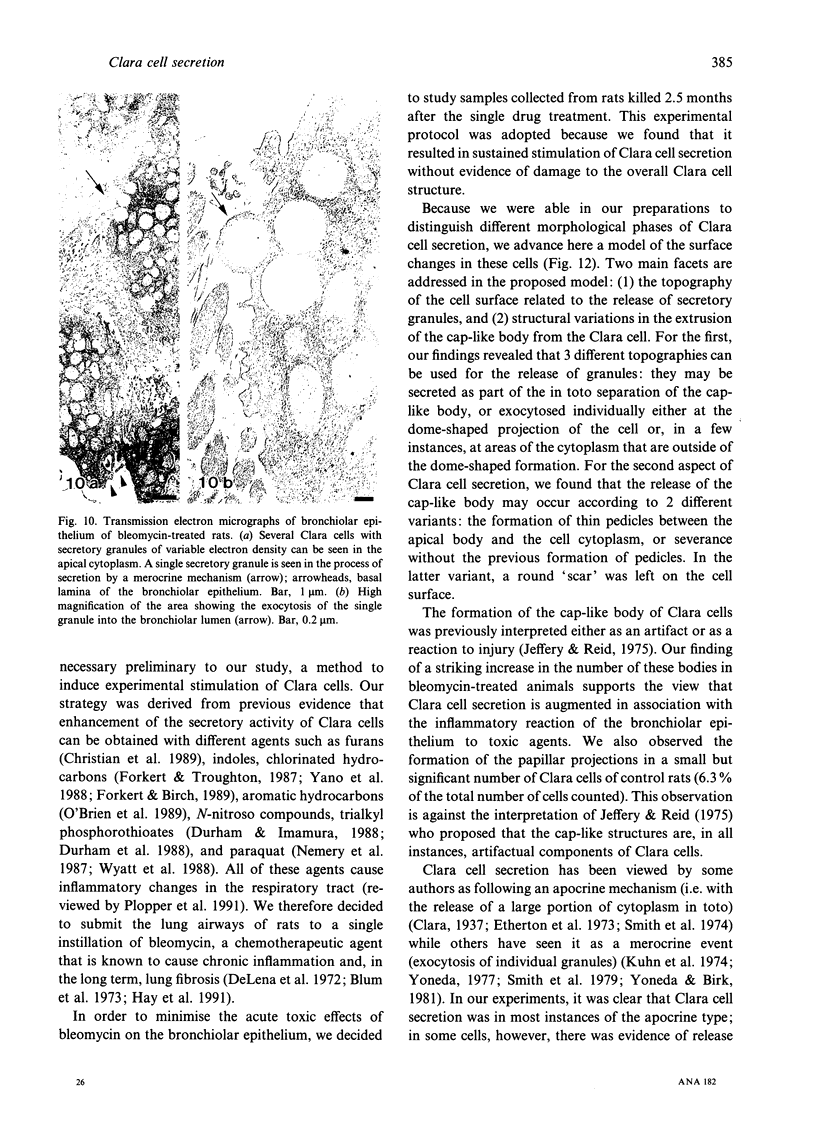
The microanatomical alterations of the surface of lung Clara cells were studied during secretion. Stimulation of Clara cells was induced in rats by chronic inflammation caused by a single intratracheal instillation of bleomycin performed 2.5 months before the animals were killed. Bleomycin treatment resulted in marked stimulation of secretion by the bronchiolar Clara cells, 31% of the Clara cells from the treated rats showing signs of active secretion whereas only 6.3% of Clara cells in control rats presented similar features. High-resolution views of lung airways were obtained by scanning electron microscopy of critical point dried tissue samples. The surface of Clara cells underwent several modifications associated with the secretory events. These alterations followed 3 major phases: (1) formation of a smooth apical dome made up of a large volume of cytoplasm; (2) progressive narrowing of this dome-like body at its base with the formation of a cap-like structure; (3) in toto release of the cytoplasmic cap-like body. In favourable views, thin pedicles linking the cap-like bodies to the remaining cytoplasm of the Clara cell were detected. In other instances, release of the cap-like body occurred without the previous formation of stalks. Secretion of intracellular granules was observed in some cells before severance of the cap-like body. It is concluded that: (1) the cap-like bodies are not artifactual features of Clara cells; (2) Clara cell secretion is both apocrine and merocrine, the former predominating; (3) chronic inflammation is associated with an increased formation and release of the secretory cap-like bodies by Clara cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguas A. P. The use of osmium tetroxide-potassium ferrocyanide as an extracellular tracer in electron microscopy. Stain Technol. 1982 Mar;57(2):69–73. doi: 10.3109/10520298209066530. [DOI] [PubMed] [Google Scholar]
- Blum R. H., Carter S. K., Agre K. A clinical review of bleomycin--a new antineoplastic agent. Cancer. 1973 Apr;31(4):903–914. doi: 10.1002/1097-0142(197304)31:4<903::aid-cncr2820310422>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Christian M. C., Wittes R. E., Leyland-Jones B., McLemore T. L., Smith A. C., Grieshaber C. K., Chabner B. A., Boyd M. R. 4-Ipomeanol: a novel investigational new drug for lung cancer. J Natl Cancer Inst. 1989 Aug 2;81(15):1133–1143. doi: 10.1093/jnci/81.15.1133. [DOI] [PubMed] [Google Scholar]
- Durham S. K., Imamura T. Morphogenesis of O,O,S-trimethyl phosphorothioate-induced pulmonary injury in mice. Toxicol Appl Pharmacol. 1988 Dec;96(3):417–428. doi: 10.1016/0041-008x(88)90002-6. [DOI] [PubMed] [Google Scholar]
- Durham S. K., Mezza L. E., Imamura T. Pulmonary endothelial and alveolar epithelial lesions induced by O,O,S-trimethyl phosphorothioate in rats. J Pathol. 1988 Jul;155(3):247–257. doi: 10.1002/path.1711550311. [DOI] [PubMed] [Google Scholar]
- Etherton J. E., Conning D. M., Corrin B. Autoradiographical and morphological evidence for apocrine secretion of dipalmitoyl lecithin in the terminal bronchiole of mouse lung. Am J Anat. 1973 Sep;138(1):11–35. doi: 10.1002/aja.1001380103. [DOI] [PubMed] [Google Scholar]
- Etherton J. E., Purchase I. F., Corrin B. Apocrine secretion in the terminal bronchiole of mouse lung. J Anat. 1979 Sep;129(Pt 2):305–322. [PMC free article] [PubMed] [Google Scholar]
- Forkert P. G., Birch D. W. Pulmonary toxicity of trichloroethylene in mice. Covalent binding and morphological manifestations. Drug Metab Dispos. 1989 Jan-Feb;17(1):106–113. [PubMed] [Google Scholar]
- Forkert P. G., Troughton K. M. Airway injury by trichloroethylene: a scanning electron microscopic study. J Pathol. 1987 Jun;152(2):119–125. doi: 10.1002/path.1711520208. [DOI] [PubMed] [Google Scholar]
- Hay J., Shahzeidi S., Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Heath D., Smith P., Biggar R. Clara cells in llamas born and living at high and low altitudes. Br J Dis Chest. 1980 Jan;74(1):75–80. [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Callaway L. A., Askin F. B. The formation of granules in the bronchiolar Clara cells of the rat. 1. Electron microscopy,. J Ultrastruct Res. 1974 Dec;49(3):387–400. doi: 10.1016/s0022-5320(74)90052-5. [DOI] [PubMed] [Google Scholar]
- Mahvi D., Bank H., Harley R. Morphology of a naphthalene-induced bronchiolar lesion. Am J Pathol. 1977 Mar;86(3):558–572. [PMC free article] [PubMed] [Google Scholar]
- Nemery B., Smith L. L., Aldridge W. N. Putrescine and 5-hydroxytryptamine accumulation in rat lung slices: cellular localization and responses to cell-specific lung injury. Toxicol Appl Pharmacol. 1987 Oct;91(1):107–120. doi: 10.1016/0041-008x(87)90198-0. [DOI] [PubMed] [Google Scholar]
- O'Brien K. A., Suverkropp C., Kanekal S., Plopper C. G., Buckpitt A. R. Tolerance to multiple doses of the pulmonary toxicant, naphthalene. Toxicol Appl Pharmacol. 1989 Jul;99(3):487–500. doi: 10.1016/0041-008x(89)90156-7. [DOI] [PubMed] [Google Scholar]
- Pack R. J., Al-Ugaily L. H., Morris G. The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat. 1981 Jan;132(Pt 1):71–84. [PMC free article] [PubMed] [Google Scholar]
- Peão M. N., Aguas A. P., Grande N. R. Cellular kinetics of inflammation in the pleural space of mice in response to the injection of exogenous particles. Exp Lung Res. 1992 Nov-Dec;18(6):863–876. doi: 10.3109/01902149209031712. [DOI] [PubMed] [Google Scholar]
- Peão M. N., Aguas A. P., de Sá C. M., Grande N. R. Structural artifacts and advantages of cytocentrifugation of cells as viewed by scanning electron microscopy. Scanning Microsc. 1992 Mar;6(1):281–285. [PubMed] [Google Scholar]
- Silva M. T., Appelberg R., Silva M. N., Macedo P. M. In vivo killing and degradation of Mycobacterium aurum within mouse peritoneal macrophages. Infect Immun. 1987 Sep;55(9):2006–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. N., Greenberg S. D., Spjut H. J. The Clara cell: a comparative ultrastructural study in mammals. Am J Anat. 1979 May;155(1):15–30. doi: 10.1002/aja.1001550103. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D., Moosavi H. The Clara cell. Thorax. 1974 Mar;29(2):147–163. doi: 10.1136/thx.29.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souma T. The distribution and surface ultrastructure of airway epithelial cells in the rat lung: a scanning electron microscopic study. Arch Histol Jpn. 1987 Oct;50(4):419–436. doi: 10.1679/aohc.50.419. [DOI] [PubMed] [Google Scholar]
- Stinson S. F., Loosli C. G. Ultrastructural evidence concerning the mode of secretion of electron-dense granules by Clara cells. J Anat. 1978 Oct;127(Pt 2):291–298. [PMC free article] [PubMed] [Google Scholar]
- Wyatt I., Soames A. R., Clay M. F., Smith L. L. The accumulation and localisation of putrescine, spermidine, spermine and paraquat in the rat lung. In vitro and in vivo studies. Biochem Pharmacol. 1988 May 15;37(10):1909–1918. doi: 10.1016/0006-2952(88)90536-9. [DOI] [PubMed] [Google Scholar]
- Yano T., Shibagaki T., Kitamura H., Kanisawa M. The mechanism of carbon tetrachloride induced pulmonary Clara cell damage: biochemical and morphologic studies. Res Commun Chem Pathol Pharmacol. 1988 Dec;62(3):483–493. [PubMed] [Google Scholar]
- Yoneda K., Birk M. G. The mode of secretion of the Clara cell in rat bronchiole: a freeze-fracture study. Exp Lung Res. 1981 Aug;2(3):177–185. doi: 10.3109/01902148109052313. [DOI] [PubMed] [Google Scholar]
- Yoneda K. Pilocarpine stimulation of the bronchiolar Clara cell secretion. Lab Invest. 1977 Nov;37(5):447–452. [PubMed] [Google Scholar]