Abstract
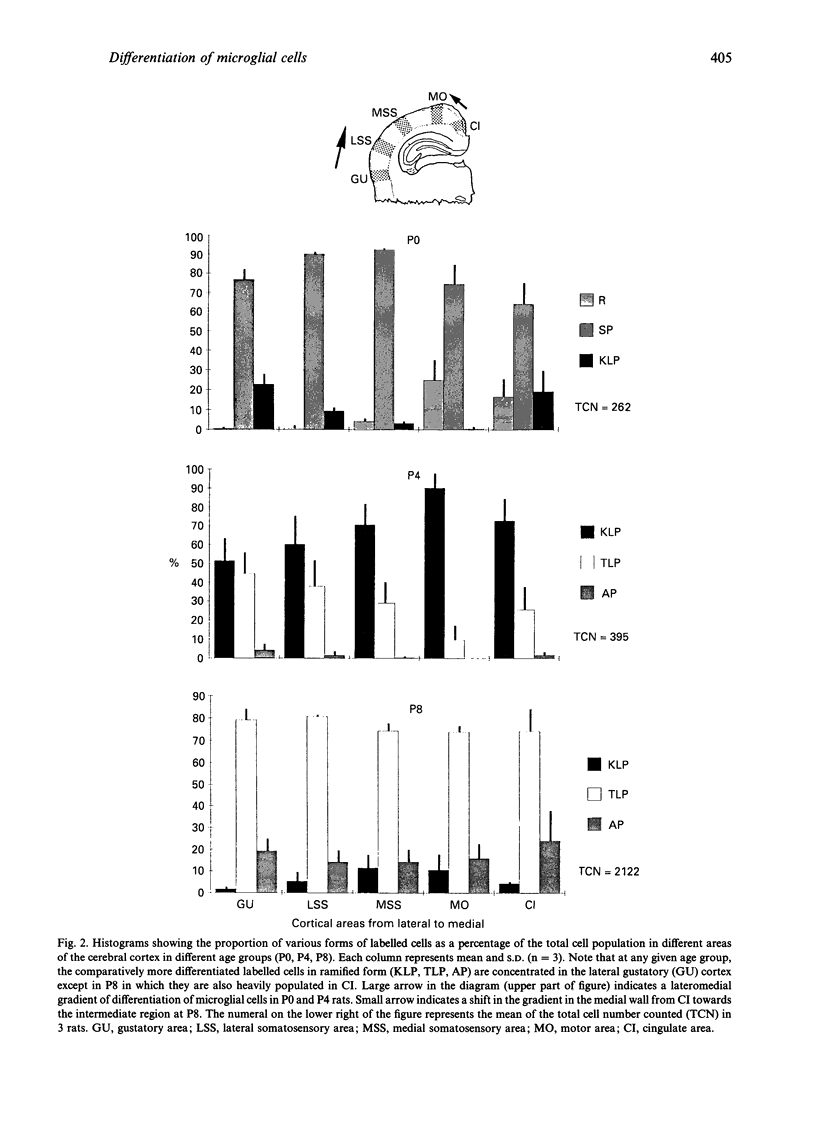
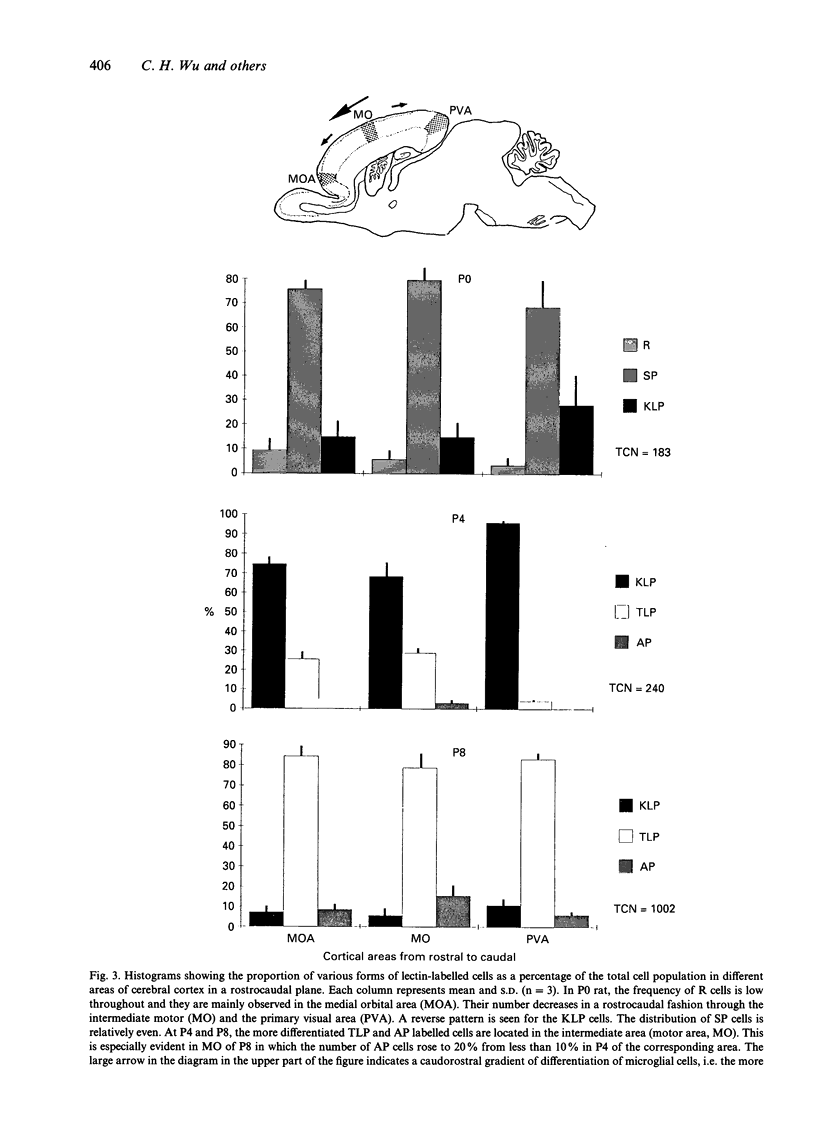
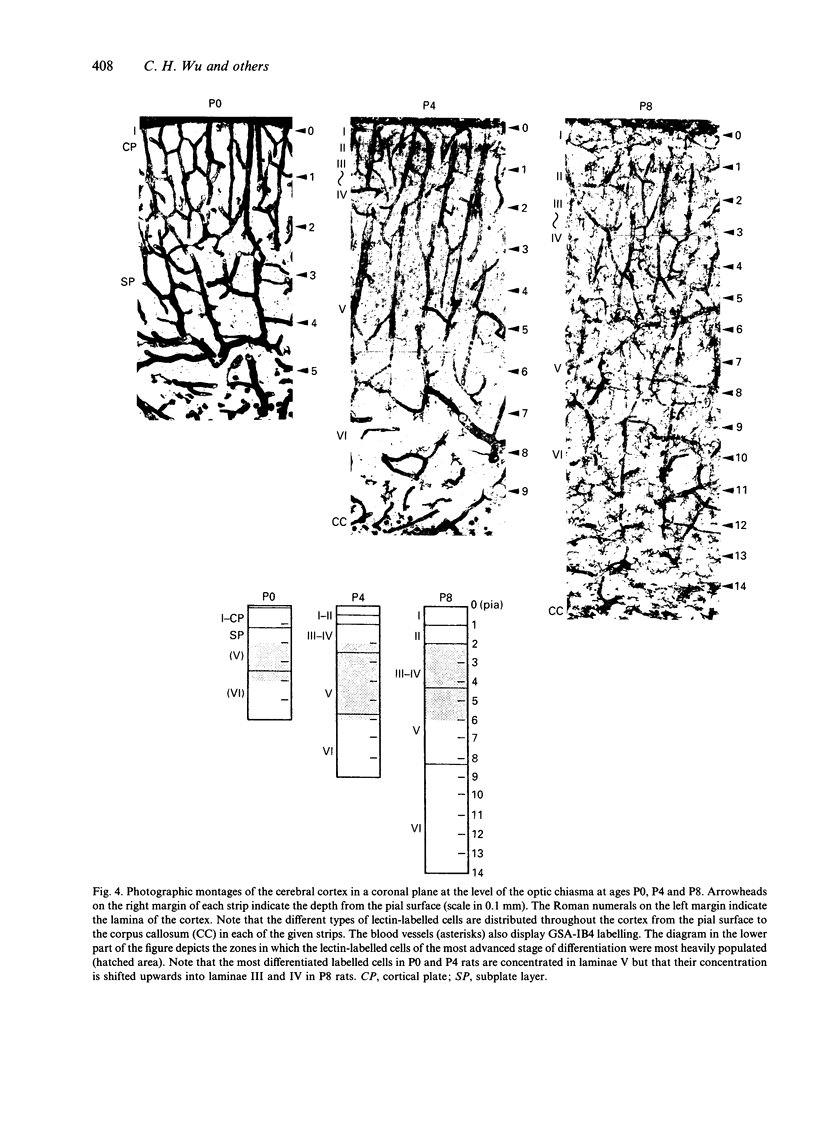
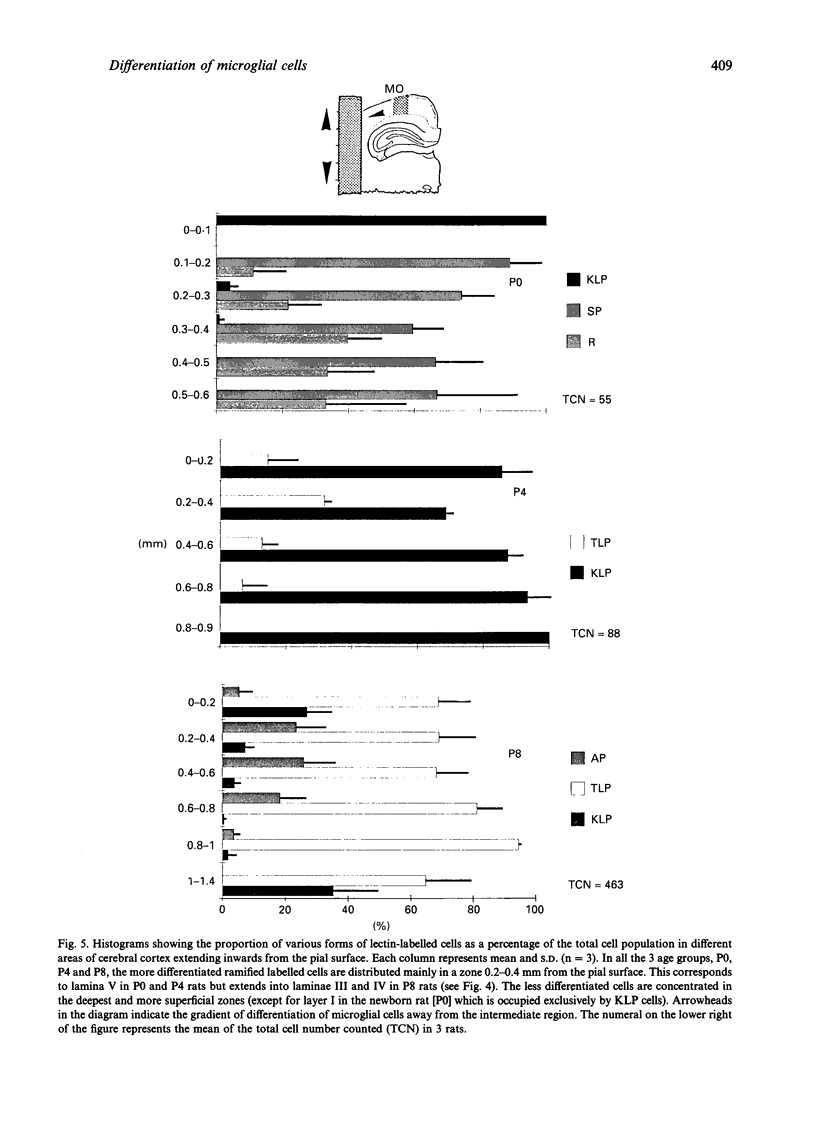
The distribution of various morphological forms of microglia stained with lectin in different regions of postnatal rat brain was examined in 2 planes of section, coronal and sagittal. In the coronal sections (mediolateral plane) taken at the level of the optic chiasma, the lectin-labelled cells were examined in the gustatory (GU), lateral somatosensory (LSS), medial somatosensory (MSS), motor (MO) and cingulate (CI) cortex. In the sagittal sections (rostrocaudal plane), the lectin-labelled cells in the medial orbital (MOA), motor (MO) and primary visual area (PVA) were studied. The cells in the motor area in coronal plane were further analysed with reference to their distribution in each of the laminae (layers). Based on the variation of their external morphology which represents different degrees of differentiation, all lectin-labelled microglial cells of the above-mentioned regions in newborn (P0) and rats aged 4 d (P4) and 8 d (P8) postnatum were classified and quantified. In the mediolateral plane of any given age group, the most differentiated ramified cells were located in GU except in P8 rats where the cells were also concentrated in CI. Of the 3 regions in the rostrocaudal plane the majority of the more differentiated ramified cells at P0 were found in the PVA but were the major cell type in MO in P4 and P8 rats. For the distribution of cells in MO, the most differentiated cells were located in the intermediate zones. It was concluded from this study that microglial cells in the developing cerebrum showed a gradient of differentiation in relation to different regions of the cerebral cortex but this appeared to vary with age. Thus in the mediolateral plane in P0 and P4 rats, the gradient extended from GU to CI in a lateromedial fashion but in P8, in the direction towards MSS from GU and CI. In the sagittal section, the gradient was directed caudorostrally in P0 rats. In P4 and P8 rats, however, the gradient was from MO to both poles (MOA, PVA). In the motor cortex, the gradient was from the intermediate zone towards the superficial and deep laminae. The gradient of differentiation of microglia may be related to the growth of the respective regions in the cerebral hemisphere but inherent genetic factors were also considered.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angevine J. B., Jr, Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961 Nov 25;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- Ashwell K. W., Holländer H., Streit W., Stone J. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2(5):437–448. doi: 10.1017/s0952523800012335. [DOI] [PubMed] [Google Scholar]
- BERRY M., ROGERS A. W., EAYRS J. T. PATTERN OF CELL MIGRATION DURING CORTICAL HISTOGENESIS. Nature. 1964 Aug 8;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- Boya J., Calvo J., Carbonell A. L. Appearance of microglial cells in the postnatal rat retina. Arch Histol Jpn. 1987 May;50(2):223–228. doi: 10.1679/aohc.50.223. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Bernet E., Soriano E., del Rio T., Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39(2):451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Fujimoto E., Miki A., Mizoguti H. Histochemical studies of the differentiation of microglial cells in the cerebral hemispheres of chick embryos and chicks. Histochemistry. 1987;87(3):209–216. doi: 10.1007/BF00492411. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kiefer R., Schoen S. W., Kreutzberg G. W. New expression of myelomonocytic antigens by microglia and perivascular cells following lethal motor neuron injury. J Neuroimmunol. 1990 May;27(2-3):121–132. doi: 10.1016/0165-5728(90)90061-q. [DOI] [PubMed] [Google Scholar]
- Hicks S. P., D'Amato C. J. Cell migrations to the isocortex in the rat. Anat Rec. 1968 Mar;160(3):619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Perry V. H., Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983 Jul;97(1):253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S., Koppel H. Transitory macrophages in the white matter of the developing visual cortex. II. Development and relations with axonal pathways. Brain Res. 1983 Dec;313(1):55–66. doi: 10.1016/0165-3806(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A. Study of the transformation of amoeboid microglial cells into microglia labelled with the isolectin Griffonia simplicifolia in postnatal rats. Acta Anat (Basel) 1991;142(2):118–125. doi: 10.1159/000147175. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Transformation of amoeboid microglial cells into microglia in the corpus callosum of the postnatal rat brain. An electron microscopical study. Arch Histol Jpn. 1985 Feb;48(1):17–25. doi: 10.1679/aohc.48.17. [DOI] [PubMed] [Google Scholar]
- Kristt D. A., Molliver M. E. Synapses in newborn rat cerebral cortex: a quantitative ultrastructural study. Brain Res. 1976 May 21;108(1):180–186. doi: 10.1016/0006-8993(76)90175-x. [DOI] [PubMed] [Google Scholar]
- Kristt D. A. Neuronal differentiation in somatosensory cortex of the rat. I. Relationship to synaptogenesis in the first postnatal week. Brain Res. 1978 Jul 21;150(3):467–486. doi: 10.1016/0006-8993(78)90814-4. [DOI] [PubMed] [Google Scholar]
- Lawson L. J., Perry V. H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res. 1970 Oct 13;23(2):167–183. doi: 10.1016/0006-8993(70)90037-5. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. VI. Postnatal development of microglial cells. Cell Tissue Res. 1982;225(3):469–485. doi: 10.1007/BF00214798. [DOI] [PubMed] [Google Scholar]
- NOBACK C. R., PURPURA D. P. Postnatal ontogenesis of neurons in cat neocortex. J Comp Neurol. 1961 Dec;117:291–307. doi: 10.1002/cne.901170303. [DOI] [PubMed] [Google Scholar]
- Rowan R. A., Maxwell D. S. Patterns of vascular sprouting in the postnatal development of the cerebral cortex of the rat. Am J Anat. 1981 Mar;160(3):247–255. doi: 10.1002/aja.1001600303. [DOI] [PubMed] [Google Scholar]
- Sanyal S., De Ruiter A. Inosine diphosphatase as a histochemical marker of retinal microvasculature, with special reference to transformation of microglia. Cell Tissue Res. 1985;241(2):291–297. doi: 10.1007/BF00217173. [DOI] [PubMed] [Google Scholar]
- Smart I. H., McSherry G. M. Growth patterns in the lateral wall of the mouse telencephalon. II. Histological changes during and subsequent to the period of isocortical neuron production. J Anat. 1982 May;134(Pt 3):415–442. [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Todd P. H., Smart I. H. Growth patterns in the lateral wall of the mouse telencephalon: III. Studies of the chronologically ordered column hypothesis of isocortical histogenesis. J Anat. 1982 Jun;134(Pt 4):633–642. [PMC free article] [PubMed] [Google Scholar]
- Wise S. P., Jones E. G. Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J Comp Neurol. 1978 Mar 15;178(2):187–208. doi: 10.1002/cne.901780202. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Wen C. Y., Shieh J. Y., Ling E. A. A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat. 1992 Dec;181(Pt 3):423–430. [PMC free article] [PubMed] [Google Scholar]




