Abstract
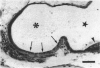
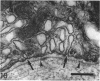
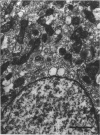
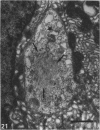
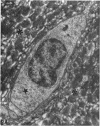
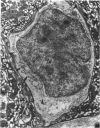
The distribution of ionocyte areas in the trout labyrinth (in the semicircular canal ampullae, crus communis and utricular vesicle) is very similar to that of areas of dark cells in mammals. In all 3 regions, ionocytes begin to develop after hatching, and are cuboid at fry stages and prismatic in juveniles and adults. On electron microscopy, 3 types of cell can be seen in adult ionocyte areas: ionocytes, associate cells and basal cells. Ionocytes possess many mitochondria, occupying approximately 28% of the cytoplasmic volume, and a well-developed tubulomembranous system which opens on the basal surface at some points. These ultrastructural features, very similar to those of chloride cells, strongly suggest that ionocytes are involved in ion transport. Unlike mammalian dark cells, there are no basal or lateral infoldings of the plasma membrane in trout ionocytes. Trout associate cells have a well developed vacuolar system, few mitochondria and bundles of cytoplasmic filaments. Although less specialised than ionocytes, they may be involved in endolymph secretion.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becerra M., Anadón R. The structure and development of the 'plana semilunata' of the labyrinth of the trout. J Anat. 1992 Apr;180(Pt 2):247–253. [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Ferrary E., Sterkers O. Production of endolymph in the semicircular canal of the frog Rana esculenta. J Physiol. 1986 Feb;371:17–28. doi: 10.1113/jphysiol.1986.sp015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche D. A., Cotton C. U., Gatzy J. T., Sulik K. K. Ultrastructural and electrophysiological maturation of the chick tegmentum vasculosum. Hear Res. 1987;25(2-3):125–139. doi: 10.1016/0378-5955(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Igarashi Y. Submicroscopic study of the vestibular dark cell area in human fetuses. Acta Otolaryngol. 1989 Jan-Feb;107(1-2):29–38. doi: 10.3109/00016488909127476. [DOI] [PubMed] [Google Scholar]
- Ishiyama E., Cutt R. A., Keels E. W. Ultrastructure of the tegmentum vasculosum and transitional zone. Ann Otol Rhinol Laryngol. 1970 Oct;79(5):998–1009. doi: 10.1177/000348947007900520. [DOI] [PubMed] [Google Scholar]
- KIMURA R., LUNDQUIST P. G., WERSAELL J. SECRETORY EPITHELIAL LININGS IN THE AMPULLAE OF THE GUINEA PIG LABYRINTH. Acta Otolaryngol. 1964 Jun;57:517–530. doi: 10.3109/00016486409137114. [DOI] [PubMed] [Google Scholar]
- Kimura R. S. Distribution, structure, and function of dark cells in the vestibular labyrinth. Ann Otol Rhinol Laryngol. 1969 Jun;78(3):542–561. doi: 10.1177/000348946907800311. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Hilding D. Vestibular endolymph-producing epithelium. Electron microscopic study of the development and histochemistry of the dark cells of the crista ampullaris. Acta Otolaryngol. 1968 Jul-Aug;66(1):120–128. doi: 10.3109/00016486809126280. [DOI] [PubMed] [Google Scholar]
- Pisam M., Prunet P., Boeuf G., Rambourg A. Ultrastructural features of chloride cells in the gill epithelium of the Atlantic salmon, Salmo salar, and their modifications during smoltification. Am J Anat. 1988 Nov;183(3):235–244. doi: 10.1002/aja.1001830306. [DOI] [PubMed] [Google Scholar]
- West R. W. Superficial warming of epoxy blocks for cutting of 25-150 m sections to be resectioned in the 40-90 nm range. Stain Technol. 1972 Jul;47(4):201–204. doi: 10.3109/10520297209116485. [DOI] [PubMed] [Google Scholar]