Abstract
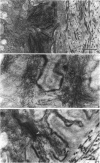
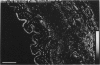
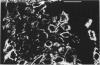
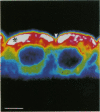
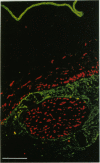
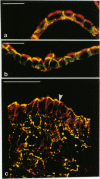
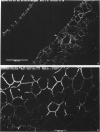
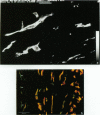
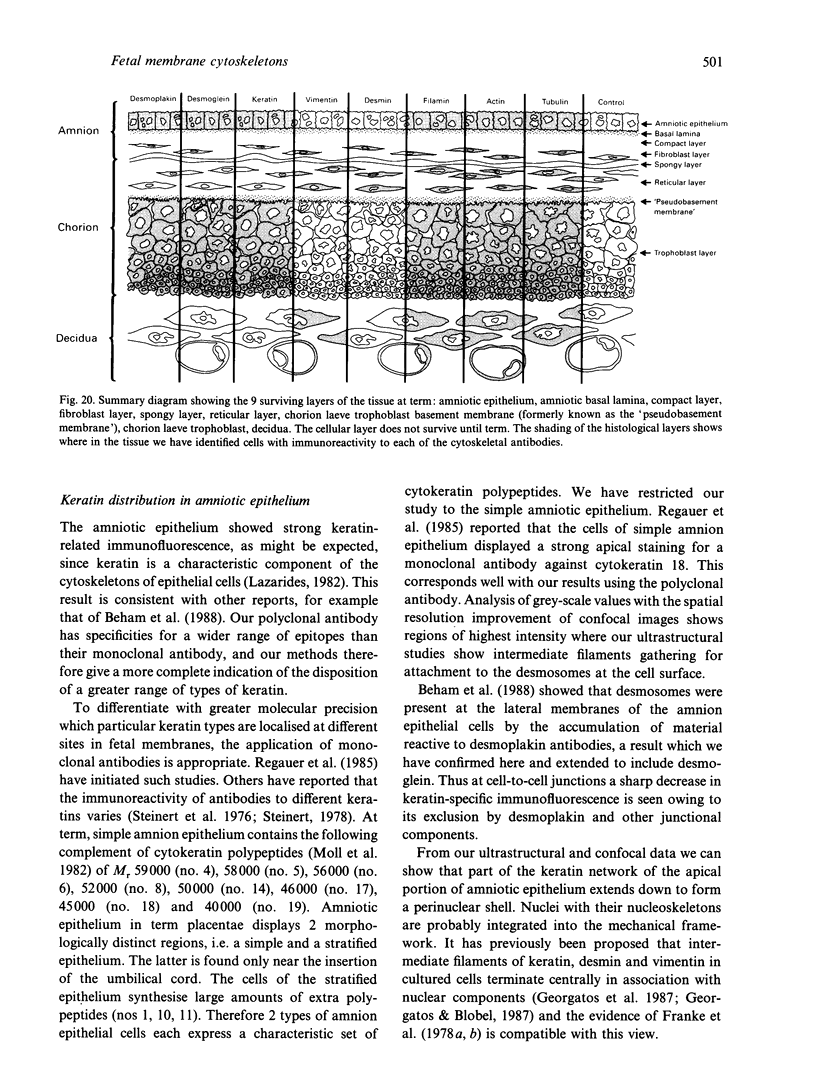
Key cytoskeletal polypeptides of human fetal membranes have been localised at subcellular level using confocal and conventional indirect immunofluorescence microscopy. Correlation with electron microscope data has allowed us to examine how cellular compartments of this multilaminar tissue maintain their mechanical integrity until the time of membrane rupture at parturition. Evidence is presented for myofibroblastic characteristics of cells in both the fibroblast and reticular layers which may therefore have tension-generating, position-adjustment and wound-healing roles in the amniochorion. Desmin and vimentin are coexpressed in these cells, but a small localised population of cells in the fibroblast layer contains vimentin alone. An interaction of cytokeratin filaments with nuclei and desmosomes of amniotic epithelium in vivo is demonstrated, indicating that nuclei of cells of ectodermal origin are integrated into a mechanical structure extending throughout the tissue as a whole. Cells of the basal 1 or 2 layers of trophoblast have been shown to have a more extensive and better integrated cytoskeletal organisation than those overlying and forming the boundary with decidua. Structures within the trophoblast, identified previously as degenerate villi, contain cells with intermediate filaments with similar immunofluorescence properties to those of the neighbouring reticular layer and thus may represent papillae that prevent shearing at this interface.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Campbell S., Allen T. D. The extracellular matrix of human amniotic epithelium: ultrastructure, composition and deposition. J Cell Sci. 1985 Nov;79:119–136. doi: 10.1242/jcs.79.1.119. [DOI] [PubMed] [Google Scholar]
- Asch H. L., Mayhew E., Lazo R. O., Asch B. B. Lipids noncovalently associated with keratins and other cytoskeletal proteins of mouse mammary epithelial cells in primary culture. Biochim Biophys Acta. 1990 Jun 20;1034(3):303–308. doi: 10.1016/0304-4165(90)90056-3. [DOI] [PubMed] [Google Scholar]
- Beham A., Denk H., Desoye G. The distribution of intermediate filament proteins, actin and desmoplakins in human placental tissue as revealed by polyclonal and monoclonal antibodies. Placenta. 1988 Sep-Oct;9(5):479–492. doi: 10.1016/0143-4004(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Berod A., Hartman B. K., Pujol J. F. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981 Jul;29(7):844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Bourne G. L. The anatomy of the human amnion and chorion. Proc R Soc Med. 1966 Nov;59(11 Pt 1):1127–1128. [PMC free article] [PubMed] [Google Scholar]
- Bradbury F. M., Ockleford C. D. A confocal and conventional epifluorescence microscope study of the intermediate filaments in chorionic villi. J Anat. 1990 Apr;169:173–187. [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Larsen P. M., Fey S. J., Celis A. Phosphorylation of keratin and vimentin polypeptides in normal and transformed mitotic human epithelial amnion cells: behavior of keratin and vimentin filaments during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1429–1434. doi: 10.1083/jcb.97.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A. C., Schneider H., Schmidt D., Parwaresch M. R. Myofibroblast as a major cellular constituent of villous stroma in human placenta. Placenta. 1985 Sep-Oct;6(5):405–415. doi: 10.1016/s0143-4004(85)80017-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982 Aug;30(1):103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Geiger B. Intermediate filaments. Looking for a function. Nature. 1987 Oct 1;329(6138):392–393. doi: 10.1038/329392a0. [DOI] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Weber K., Geisler N., Blobel G. Binding of two desmin derivatives to the plasma membrane and the nuclear envelope of avian erythrocytes: evidence for a conserved site-specificity in intermediate filament-membrane interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6780–6784. doi: 10.1073/pnas.84.19.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. Modulation of keratin intermediate filament assembly by single amino acid exchanges in the consensus sequence at the C-terminal end of the rod domain. J Cell Sci. 1991 Jun;99(Pt 2):351–362. doi: 10.1242/jcs.99.2.351. [DOI] [PubMed] [Google Scholar]
- Henderson D., Weber K. Immuno-electron microscopical identification of the two types of intermediate filaments in established epithelial cells. Exp Cell Res. 1981 Apr;132(2):297–311. doi: 10.1016/0014-4827(81)90106-3. [DOI] [PubMed] [Google Scholar]
- Hubbard B. D., Lazarides E. Copurification of actin and desmin from chicken smooth muscle and their copolymerization in vitro to intermediate filaments. J Cell Biol. 1979 Jan;80(1):166–182. doi: 10.1083/jcb.80.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987 Aug 13;328(6131):649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Schmid E., Bürki K., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Khong T. Y., Lane E. B., Robertson W. B. An immunocytochemical study of fetal cells at the maternal-placental interface using monoclonal antibodies to keratins, vimentin and desmin. Cell Tissue Res. 1986;246(1):189–195. doi: 10.1007/BF00219017. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments. Getting under the skin. Nature. 1991 Nov 28;354(6351):264–264. doi: 10.1038/354264a0. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schmid E., Jorcano J. L., Franke W. W. De novo synthesis and specific assembly of keratin filaments in nonepithelial cells after microinjection of mRNA for epidermal keratin. Cell. 1983 Apr;32(4):1125–1137. doi: 10.1016/0092-8674(83)90296-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D., Hsi B. L., Wakely J., Badley R. A., Whyte A., Faulk W. P. Propidium iodide as a nuclear marker in immunofluorescence. I. Use with tissue and cytoskeleton studies. J Immunol Methods. 1981;43(3):261–267. doi: 10.1016/0022-1759(81)90173-3. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D., Wakely J., Badley R. A. Morphogenesis of human placental chorionic villi: cytoskeletal, syncytioskeletal and extracellular matrix proteins. Proc R Soc Lond B Biol Sci. 1981 Jul 14;212(1188):305–316. doi: 10.1098/rspb.1981.0041. [DOI] [PubMed] [Google Scholar]
- Parrish E. P., Steart P. V., Garrod D. R., Weller R. O. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol. 1987 Nov;153(3):265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Steven A. C., Steinert P. M. The coiled-coil molecules of intermediate filaments consist of two parallel chains in exact axial register. Biochem Biophys Res Commun. 1985 Mar 29;127(3):1012–1018. doi: 10.1016/s0006-291x(85)80045-0. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Regauer S., Franke W. W., Virtanen I. Intermediate filament cytoskeleton of amnion epithelium and cultured amnion epithelial cells: expression of epidermal cytokeratins in cells of a simple epithelium. J Cell Biol. 1985 Apr;100(4):997–1009. doi: 10.1083/jcb.100.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Direct visualization of the 10-nm (100-A)-filament network in whole and enucleated cultured cells. J Cell Sci. 1978 Jun;31:393–409. doi: 10.1242/jcs.31.1.393. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]