Abstract
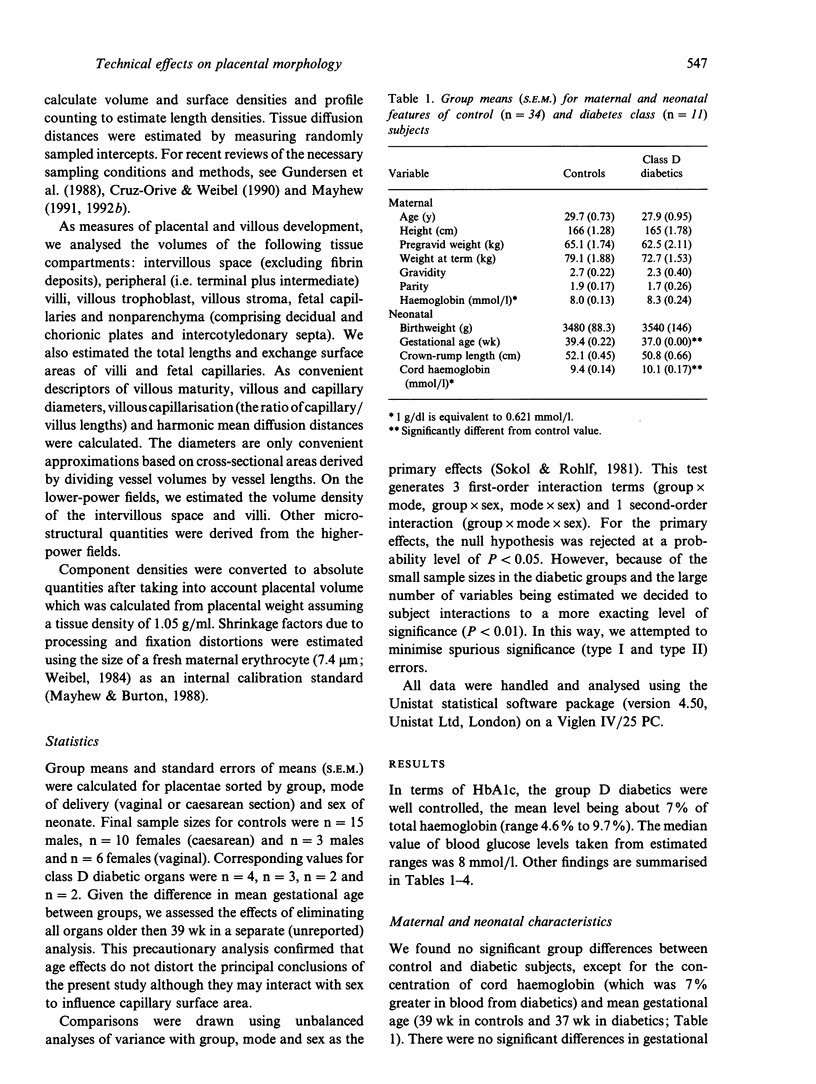
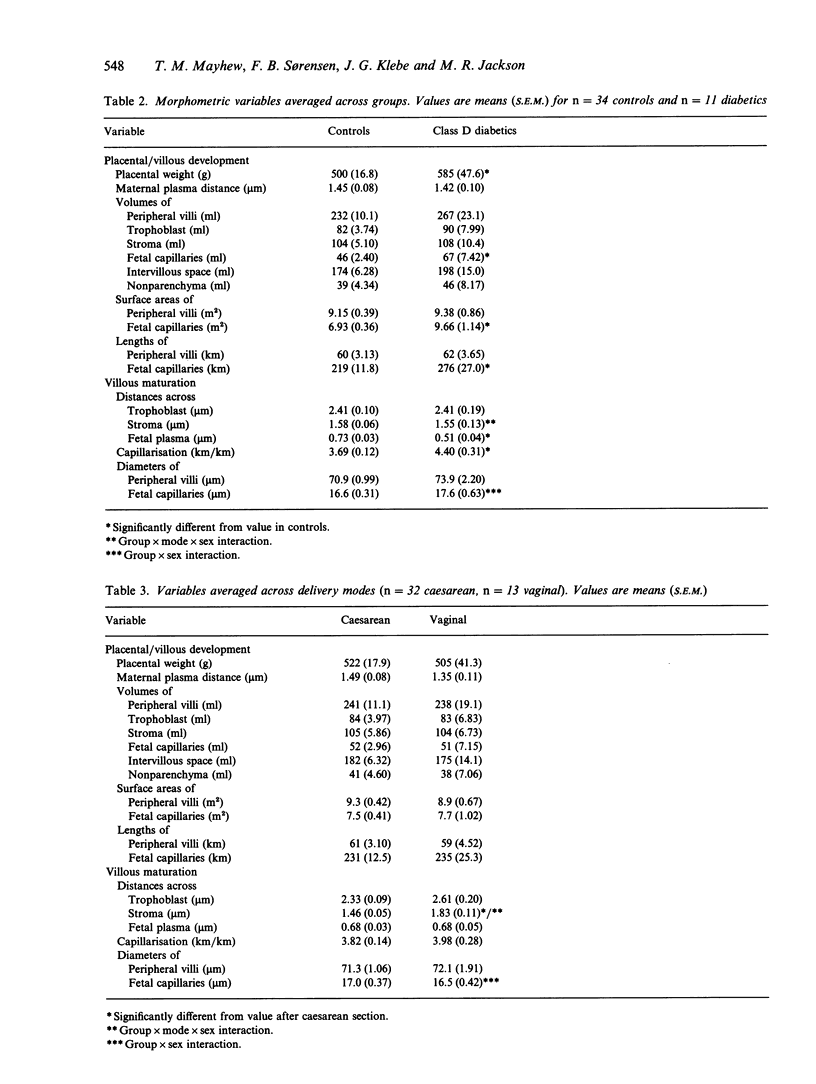
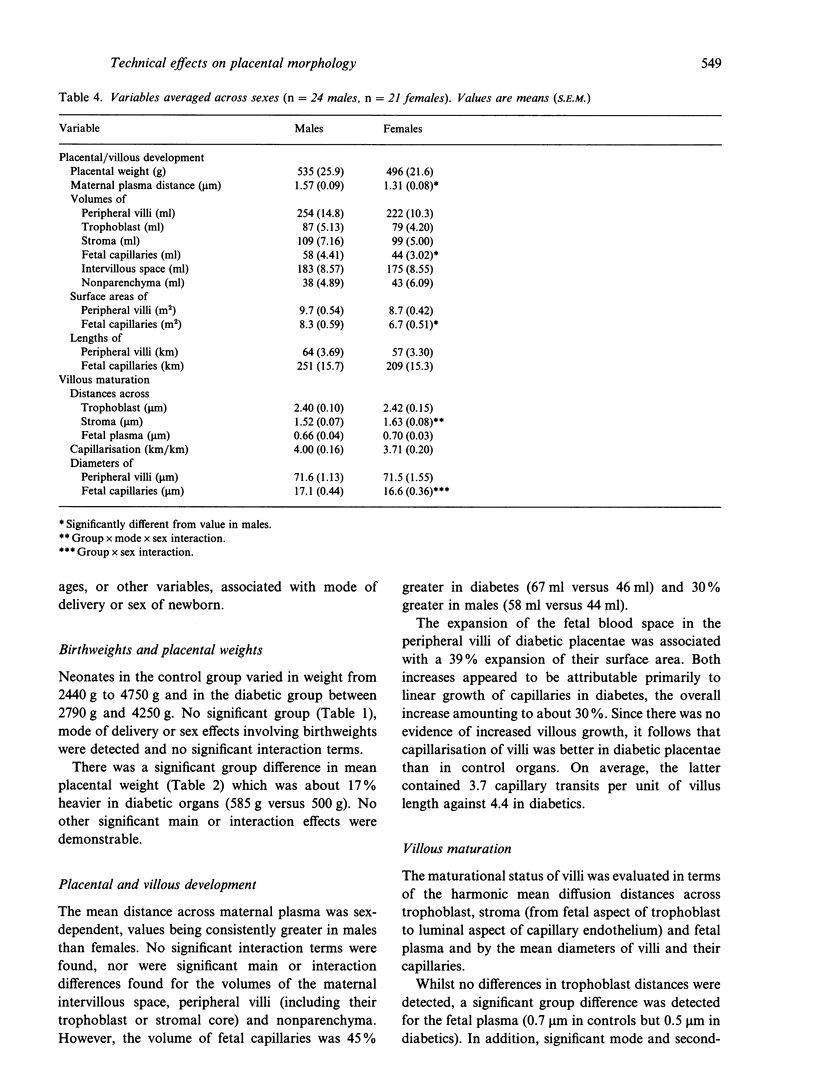
Placentae from control and diabetic subjects were analysed using stereological techniques in order to assess the effects of mode of delivery (vaginal versus caesarean) and sex of neonate on parenchymal morphology. Effects were assessed using indices of peripheral villous and fetal capillary growth, villous maturity, extent of maternal intervillous space and thickness of intervascular tissue layers. Placentae were from pregnancies (37-42 wk) which were either uncomplicated (control group) or complicated by diabetes mellitus (diabetic group, White class D) which was reasonably well controlled in terms of glucose and glycated haemoglobin levels. Neonatal and placental weights were recorded and placentae sampled in a systematic random fashion. Fields of view on formalin-fixed, paraffin-embedded sections were analysed to obtain estimates of volumes, surface areas, lengths and diffusion (harmonic mean) distances. Comparisons were drawn using 3-way analyses of variance with group, mode of delivery and sex as the principal effects. The mean length of gestation was 2 wk longer in controls (39 versus 37 wk). Despite this, mean birth weight was similar (3.5 kg) in control and diabetic groups. Moreover, diabetic placentae were 17% heavier and showed shorter fetal plasma distances (30%) and larger fetal capillaries (volume 45%, surface 39% and length 30% greater). Mode of delivery had significant main and interaction effects on stromal diffusion distance (25% greater in vaginal deliveries) and an interaction effect on fetal capillary volume. Sex had significant main effects on the maternal plasma distance (21% greater in males) and capillary volume (30% bigger in males) and an interaction effect on placental weight and mean capillary diameter.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aherne W., Dunnill M. S. Quantitative aspects of placental structure. J Pathol Bacteriol. 1966 Jan;91(1):123–139. doi: 10.1002/path.1700910117. [DOI] [PubMed] [Google Scholar]
- Aladjem S. Morphologic aspects of the placenta in gestational diabetes seen by phase-contrast microscopy. An anatomicoclinical correlation. Am J Obstet Gynecol. 1967 Oct 1;99(3):341–349. doi: 10.1016/s0002-9378(16)34541-0. [DOI] [PubMed] [Google Scholar]
- BURSTEIN R., BERNS A. W., HIRATA Y., BLUMENTHAL H. T. A comparative histo- and immunopathological study of the placenta in diabetes mellitus and in erythroblastosis fetalis. Am J Obstet Gynecol. 1963 May 1;86:66–76. doi: 10.1016/0002-9378(63)90077-2. [DOI] [PubMed] [Google Scholar]
- Ballew C., Haas J. D. Hematologic evidence of fetal hypoxia among newborn infants at high altitude in Bolivia. Am J Obstet Gynecol. 1986 Jul;155(1):166–169. doi: 10.1016/0002-9378(86)90104-3. [DOI] [PubMed] [Google Scholar]
- Björk O., Persson B. Placental changes in relation to the degree of metabolic control in diabetes mellitus. Placenta. 1982 Oct-Dec;3(4):367–378. doi: 10.1016/s0143-4004(82)80030-1. [DOI] [PubMed] [Google Scholar]
- Björk O., Persson B. Villous structure in different parts of the cotyledon in placentas of insulin-dependent diabetic women. A morphometric study. Acta Obstet Gynecol Scand. 1984;63(1):37–43. doi: 10.3109/00016348409156271. [DOI] [PubMed] [Google Scholar]
- Boyd P. A., Scott A., Keeling J. W. Quantitative structural studies on placentas from pregnancies complicated by diabetes mellitus. Br J Obstet Gynaecol. 1986 Jan;93(1):31–35. doi: 10.1111/j.1471-0528.1986.tb07809.x. [DOI] [PubMed] [Google Scholar]
- Burton G. J., Ingram S. C., Palmer M. E. The influence of mode of fixation on morphometrical data derived from terminal villi in the human placenta at term: a comparison of immersion and perfusion fixation. Placenta. 1987 Jan-Feb;8(1):37–51. doi: 10.1016/0143-4004(87)90038-5. [DOI] [PubMed] [Google Scholar]
- CLAVERO J. A., BOTELLALLUSIA J. Measurement of the villus surface in normal and pathologic placentas. Am J Obstet Gynecol. 1963 May 15;86:234–240. doi: 10.1016/0002-9378(63)90436-8. [DOI] [PubMed] [Google Scholar]
- Cruz-Orive L. M., Weibel E. R. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990 Apr;258(4 Pt 1):L148–L156. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- Diamond M. P., Salyer S. L., Vaughn W. K., Cotton R., Boehm F. H. Reassessment of White's classification and Pedersen's prognostically bad signs of diabetic pregnancies in insulin-dependent diabetic pregnancies. Am J Obstet Gynecol. 1987 Mar;156(3):599–604. doi: 10.1016/0002-9378(87)90060-3. [DOI] [PubMed] [Google Scholar]
- Emmrich P., Fuchs U., Heinke P., Jutzi E., Gödel E. The epithelial and capillary basal laminae of the placenta in maternal diabetes mellitus. Lab Invest. 1976 Jul;35(1):87–92. [PubMed] [Google Scholar]
- Fox H. Pathology of the placenta in maternal diabetes mellitus. Obstet Gynecol. 1969 Dec;34(6):792–798. [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Haust M. D. Maternal diabetes mellitus--effects on the fetus and placenta. Monogr Pathol. 1981;(22):201–285. [PubMed] [Google Scholar]
- Jackson M. R., Mayhew T. M., Boyd P. A. Quantitative description of the elaboration and maturation of villi from 10 weeks of gestation to term. Placenta. 1992 Jul-Aug;13(4):357–370. doi: 10.1016/0143-4004(92)90060-7. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Mayhew T. M., Haas J. D. Morphometric studies on villi in human term placentae and the effects of altitude, ethnic grouping and sex of newborn. Placenta. 1987 Sep-Oct;8(5):487–495. doi: 10.1016/0143-4004(87)90077-4. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Mayhew T. M., Haas J. D. The volumetric composition of human term placentae: altitudinal, ethnic and sex differences in Bolivia. J Anat. 1987 Jun;152:173–187. [PMC free article] [PubMed] [Google Scholar]
- Jones C. J., Fox H. An ultrastructural and ultrahistochemical study of the placenta of the diabetic woman. J Pathol. 1976 Jun;119(2):91–99. doi: 10.1002/path.1711190203. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Fox H. Placental changes in gestational diabetes. An ultrastructural study. Obstet Gynecol. 1976 Sep;48(3):274–280. [PubMed] [Google Scholar]
- Klebe J. G., Ingomar C. J. Placental transfusion in infants of diabetic mothers elucidated by placental residual blood volume. Acta Paediatr Scand. 1974 Jan;63(1):59–64. doi: 10.1111/j.1651-2227.1974.tb04349.x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992 May;21(5):313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Burton G. J. Methodological problems in placental morphometry: apologia for the use of stereology based on sound sampling practice. Placenta. 1988 Nov-Dec;9(6):565–581. doi: 10.1016/0143-4004(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Jackson M. R., Haas J. D. Oxygen diffusive conductances of human placentae from term pregnancies at low and high altitudes. Placenta. 1990 Nov-Dec;11(6):493–503. doi: 10.1016/s0143-4004(05)80195-x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M. The new stereological methods for interpreting functional morphology from slices of cells and organs. Exp Physiol. 1991 Sep;76(5):639–665. doi: 10.1113/expphysiol.1991.sp003533. [DOI] [PubMed] [Google Scholar]
- Stringer B. M., Wynford-Thomas D., Williams E. D. Physical randomization of tissue architecture: an alternative to systemic sampling. J Microsc. 1982 May;126(Pt 2):179–182. doi: 10.1111/j.1365-2818.1982.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Histomorphometry of the human placenta in Class B diabetes mellitus. Placenta. 1983 Jan-Apr;4(1):1–12. doi: 10.1016/s0143-4004(83)80012-5. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Histomorphometry of the human placenta in Class C diabetes mellitus. Placenta. 1985 Jan-Feb;6(1):69–81. doi: 10.1016/s0143-4004(85)80034-5. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Histomorphometry of the placenta of the diabetic women: class A diabetes mellitus. Placenta. 1981 Jul-Sep;2(3):241–251. doi: 10.1016/s0143-4004(81)80007-0. [DOI] [PubMed] [Google Scholar]
- WHITE P. Pregnancy complicating diabetes. Am J Med. 1949 Nov;7(5):609–616. doi: 10.1016/0002-9343(49)90382-4. [DOI] [PubMed] [Google Scholar]
- Widness J. A., Susa J. B., Garcia J. F., Singer D. B., Sehgal P., Oh W., Schwartz R., Schwartz H. C. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest. 1981 Mar;67(3):637–642. doi: 10.1172/JCI110078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACKS S. I., BLAZAR A. S. CHORIONIC VILLI IN NORMAL PREGNANCY, PRE-ECLAMPTIC TOXEMIA, ERYTHROBLASTOSIS, AND DIABETES MELLITUS. A LIGHT- AND ELECTRON-MICROSCOPE STUDY. Obstet Gynecol. 1963 Aug;22:149–167. [PubMed] [Google Scholar]


