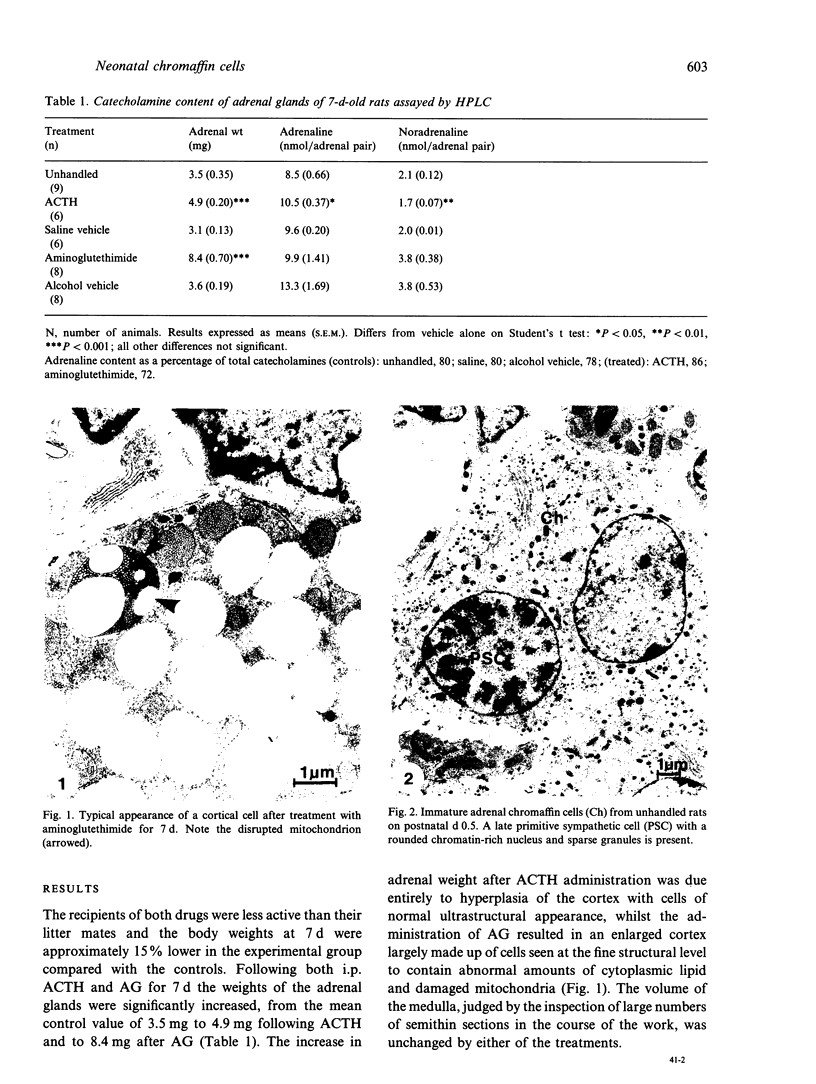
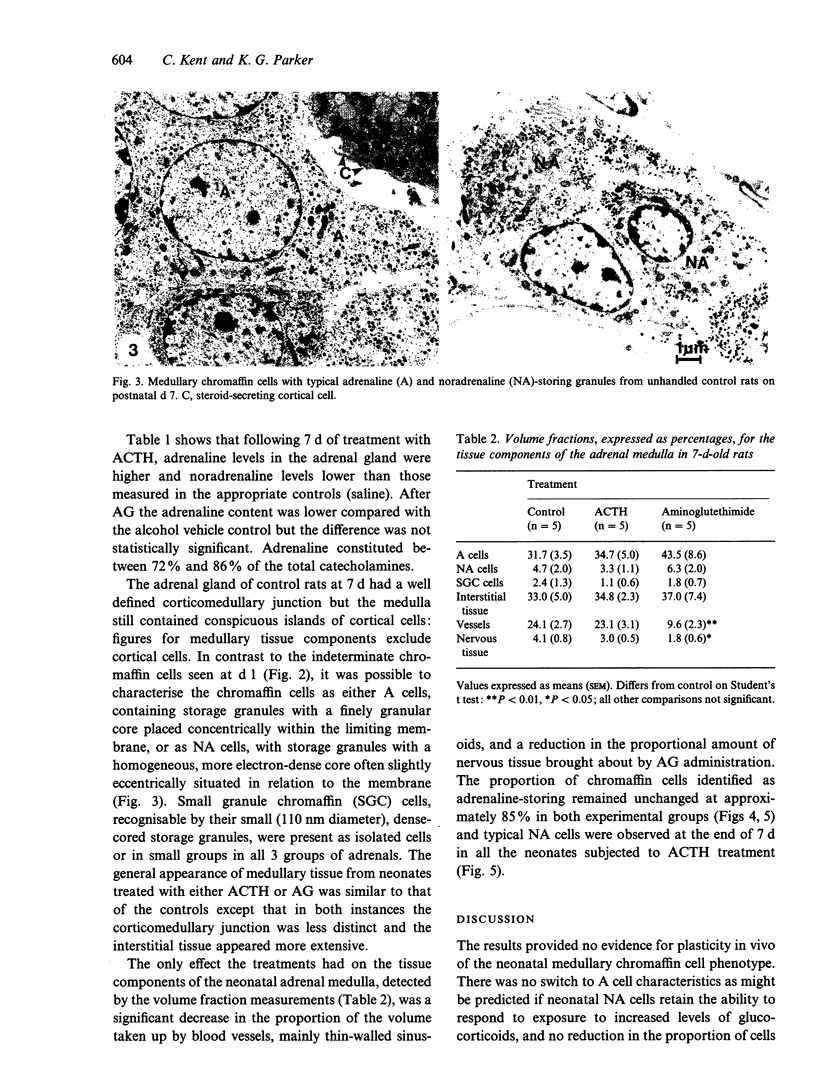
Abstract
The rat adrenal medulla is immature at birth, composed of phaeochromoblasts and undifferentiated chromaffin cells, but by 7 d postnatally morphologically distinct adrenaline-storing (A) and noradrenaline-storing (NA) cells can be distinguished in the adult proportions of approximately 80-85% A and 15-20% NA cells. Glucocorticoid hormones are known to play an important role in the initial expression and maintenance of phenylethanolamine N-methyl transferase (PNMT), the enzyme characteristic of A cells. The purpose of the study was to investigate the effects of glucocorticoids on the establishment of the A and NA cell phenotype in vivo during the first postnatal week. Neonatal rats were treated from postnatal d 1 to 7 either with ACTH to increase circulating levels of glucocorticoids or with aminoglutethimide to reduce blood glucocorticoids. On postnatal d 7 the volume fractions of A and NA cells in the adrenal medulla were estimated and the amounts of stored adrenaline and noradrenaline determined by HPLC and compared with untreated controls. Adrenaline levels were increased following ACTH treatment and there was an apparent decrease after aminoglutethimide which was not statistically significant. There was cytological evidence of the effects of ACTH and aminoglutethimide on the adrenal cortex but no resultant effect on medullary cell morphology. A cells remained predominant with NA cells making up approximately 15% of chromaffin cells, suggesting that any effects of altered glucocorticoid levels were confined to a modulation of adrenaline synthesis by a morphologically unchanged chromaffin cell population.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

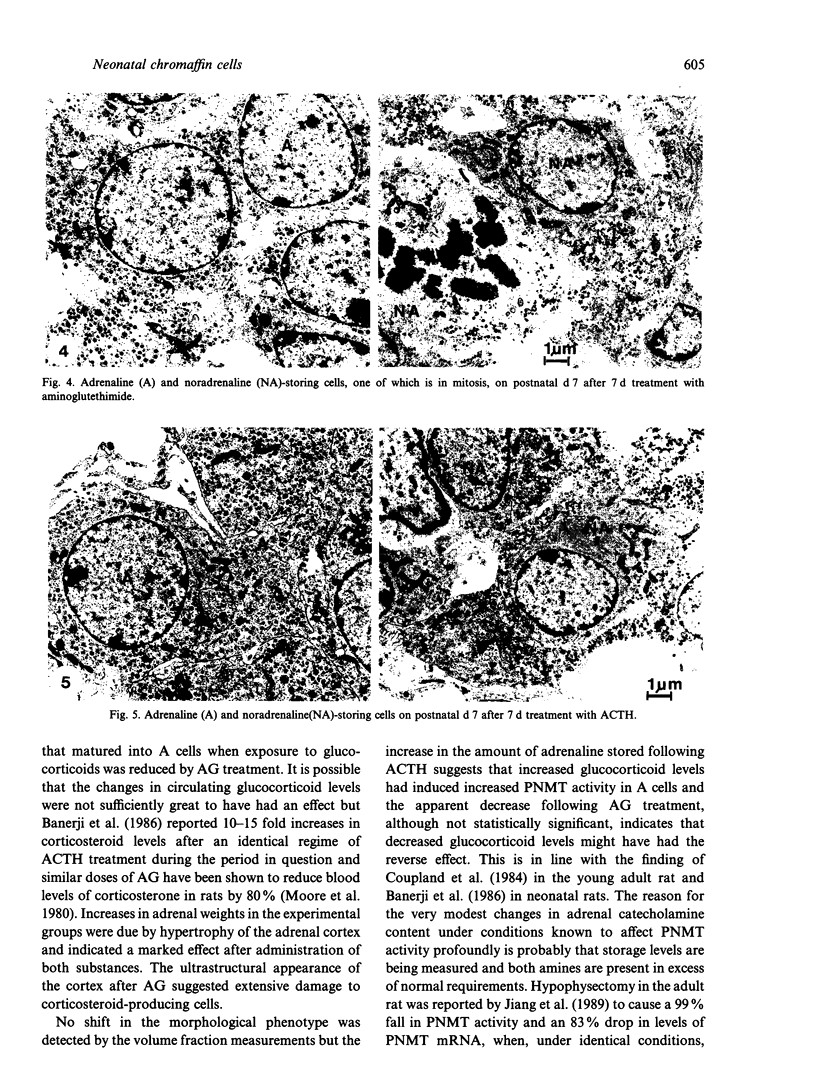


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji T. K., Callas G., Meyer W. J., Rassoli A. ACTH increases adrenal medullary PNMT activity in neonatal rats. Life Sci. 1986 Jan 27;38(4):343–349. doi: 10.1016/0024-3205(86)90081-0. [DOI] [PubMed] [Google Scholar]
- Bohn M. C., Goldstein M., Black I. B. Role of glucocorticoids in expression of the adrenergic phenotype in rat embryonic adrenal gland. Dev Biol. 1981 Feb;82(1):1–10. doi: 10.1016/0012-1606(81)90423-1. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Dagerlind A., Schalling M., Wikstróm A. C., Okret S., Gustafsson J. A., Goldstein M., Hökfelt T. The glucocorticoid receptor in the adrenal gland is localized in the cytoplasm of adrenaline cells. Acta Physiol Scand. 1989 Dec;137(4):559–560. doi: 10.1111/j.1748-1716.1989.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Coupland R. E., Tomlinson A., Crowe J., Brindley D. N. Effects of hypophysectomy and metyrapone on the catecholamine content and volumes of adrenaline- and noradrenaline-storing cells in the rat adrenal medulla. J Endocrinol. 1984 Jun;101(3):345–352. doi: 10.1677/joe.0.1010345. [DOI] [PubMed] [Google Scholar]
- Coupland R. E., Tomlinson A. The development and maturation of adrenal medullary chromaffin cells of the rat in vivo: a descriptive and quantitative study. Int J Dev Neurosci. 1989;7(5):419–438. doi: 10.1016/0736-5748(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Jiang W., Uht R., Bohn M. C. Regulation of phenylethanolamine N-methyltransferase (PNMT) mRNA in the rat adrenal medulla by corticosterone. Int J Dev Neurosci. 1989;7(5):513–520. doi: 10.1016/0736-5748(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Michelsohn A. M., Anderson D. J. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron. 1992 Mar;8(3):589–604. doi: 10.1016/0896-6273(92)90285-l. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Penney D. P., Averill K. T. Fine structural and biochemical effects of aminoglutethimide and o,p'-DDD on rat adrenocortical carcinoma 494 and adrenals. Anat Rec. 1980 Sep;198(1):113–124. doi: 10.1002/ar.1091980109. [DOI] [PubMed] [Google Scholar]
- Olson L. Fluorescence histochemical evidence for axonal growth and secretion from transplanted adrenal medullary tissue. Histochemie. 1970;22(1):1–7. doi: 10.1007/BF00310543. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstad A. A., Coupland R. E., Parker T. R., Goldstein M. Immunohistochemical and biochemical study on the development of the noradrenaline- and adrenaline-storing cells of the adrenal medulla of the rat. Cell Tissue Res. 1985;242(2):233–243. doi: 10.1007/BF00214536. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Adrenaline synthesis: control by the pituitary gland and adrenal glucocorticoids. Science. 1965 Dec 10;150(3702):1464–1465. doi: 10.1126/science.150.3702.1464. [DOI] [PubMed] [Google Scholar]
- von Dalnok G. K., Menssen H. D. A quantitative electron microscopic study of the effect of glucocorticoids in vivo on the early postnatal differentiation of paraneuronal cells in the carotid body and the adrenal medulla of the rat. Anat Embryol (Berl) 1986;174(3):307–319. doi: 10.1007/BF00698781. [DOI] [PubMed] [Google Scholar]