Abstract
To address the role of phospholipids in the topological organization of polytopic membrane proteins, the function and assembly of lactose permease (LacY) was studied in mutants of Escherichia coli lacking phosphatidylethanolamine (PE). PE is required for the proper conformation and active transport function of LacY. The N-terminal half of LacY assembled in PE-lacking cells adopts an inverted topology in which normally non-translocated domains are translocated and vice versa. Post-assembly synthesis of PE triggers a conformational change, resulting in a lipid-dependent recovery of normal conformation and topology of at least one LacY subdomain accompanied by restoration of active transport. These results demonstrate that membrane protein topology once attained can be changed in a reversible manner in response to alterations in phospholipid composition, and may be subject to post-assembly proofreading to correct misfolded structures.
Keywords: lactose permease/membrane protein assembly/phosphatidylethanolamine/phospholipid/topology
Introduction
Although considerable progress has been made in understanding the assembly of multispanning membrane proteins (Bernstein, 2000; Dalbey et al., 2000), the precise molecular events involved in the folding of proteins into the membrane are not well defined. Investigation focusing on the role of amino acid sequence in governing the assembly of membrane proteins has predominated, while the role phospholipids play as structural determinants for the correct insertion, folding and topology of membrane proteins has been largely ignored. Therefore, to date there is little understanding of, or ability to predict, how membrane protein topogenesis occurs in a given phospholipid environment.
Determinants of membrane protein topology and the mechanism of membrane protein insertion in Escherichia coli have been studied mainly for native proteins containing one or two transmembrane domains (TMs) (de Gier et al., 1998) and chimeric derivatives of a few polytopic membrane proteins (Gafvelin and von Heijne, 1994; Kim et al., 1994). Only one study focused on the interaction of positively charged cytoplasmic domains of membrane proteins with the headgroups of anionic phospholipids as a topological determinant (van Klompenburg et al., 1997). Can specific lipids influence the topological organization of membrane proteins? Is protein topology static or is it dynamic with respect to changes in membrane lipid composition? Is membrane protein sequence ‘written’ for a given membrane environment?
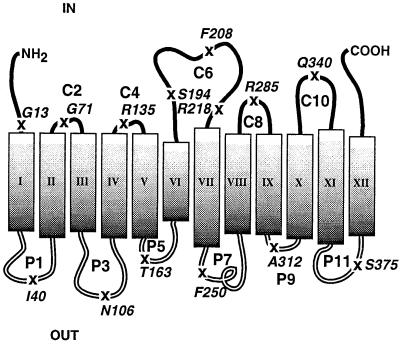
To investigate the influence of lipids on topogenesis, we have focused on the lactose permease (LacY) of E.coli, a structural paradigm for the major facilitator superfamily (MFS) of secondary transport proteins (Saier et al., 1999). Members of this family are diverse in substrate specificity yet show significant homology in function, sequence and structure. These transporters are characterized by a 12-TM topology divided into two six-helix segments connected by a long cytoplasmic loop (Figure 1). Common to many sugar permeases, including LacY, is the very hydrophilic TM VII that is not predicted by membrane topology algorithms (Efremov and Vergoten, 1996) but has been verified biochemically (Calamia and Manoil, 1990; Wolin and Kaback, 1999). The mechanism by which the TMs are oriented and the connecting extra-membrane domains are folded is not understood.
Fig. 1. Secondary structural model of LacY with indicated modifications in the linear sequence. The figure is based on the most recent putative topology organization of LacY (Kaback et al., 2001). Rectangles indicate putative helical TMs numbered sequentially in roman numerals from the N-terminus (NH2) to the C-terminus (COOH). The hydrophilic loops connecting the TMs are numbered sequentially and their putative topological disposition in PE-containing cells is indicated by the prefix ‘C’ for cytoplasmic (IN) or ‘P’ for periplasmic (OUT). The position of single Cys replacements (see Table I) in a Cys-less derivative of LacY is indicated by an ‘X’, and the single-letter amino acid code of the replaced amino acid followed by the residue number.
Phosphatidylethanolamine (PE) acts as a non-protein molecular chaperone in the folding of LacY by the programming of ‘conformational memory’ during assembly (Bogdanov et al., 1996, 1999; Bogdanov and Dowhan, 1998). This zwitterionic and major phospholipid of E.coli is required for proper assembly and full function of LacY. LacY couples the downhill movement of a proton with the uphill movement of substrate in a symport mechanism to drive active transport. However, LacY assembled in a mutant of E.coli lacking PE cannot accumulate substrate against a concentration gradient, but can still facilitate substrate transport. Bioenergetic properties of this mutant are not affected (Bogdanov and Dowhan, 1995). The loss of full function correlates with a structural alteration in the periplasmic domain P7, as indicated by loss of recognition by the conformation sensitive monoclonal antibody (mAb) 4B1 (Sun et al., 1996). PE is required either during de novo assembly in vivo or during refolding of partially denatured LacY in vitro, but is not required once ‘native structure’ has been attained. PE is not required for membrane insertion, but if added after membrane insertion it facilitates the final structural maturation of LacY as determined by conformation specific mAb4B1. Therefore, PE appears to facilitate the assembly of LacY by affecting the folding of non-native intermediates late in the maturation process.
In order to establish the molecular basis for LacY dysfunction and misfolding in PE-lacking cells, we report a systematic comparative study of the transmembrane topology of LacY assembled in PE-containing or PE-lacking cells. In wild-type cells, membrane protein assembly occurs in the presence of abundant PE (75% of total phospholipid), making it difficult to discriminate between phospholipid-dependent and -independent assembly events. By using mutants lacking PE, a lipid-dependent assembly step for LacY was identified along the pathway to attainment of native structure. This lipid requirement differs from the general solvent effect of lipids and shows specificity for PE. Our results are consistent with a topological inversion of the first half of LacY through domain C6 when LacY is assembled in membranes lacking PE. The topological inversion of at least domain C6 can be reversed by the addition of PE after membrane insertion and is accompanied by a restoration of active transport function mediated by LacY. This is the first report of a dual membrane protein topology that is interchangeable post-insertionally in response to changes in phospholipid composition. These results implicate phospholipids as specific participants in determining membrane protein organization and suggest that regulation of membrane protein function can occur by topology ‘switching’ in response to changes in phospholipid environment.
Results
Rationale and methodology for determination of domain topology
The topology of hydrophilic loops connecting TMs of LacY was established by the selective biotinylation [3- (N-maleimidylpropionyl) biocytin (MPB)] or alkylation [4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS)] of single cysteines exposed either to the periplasmic face of whole cells or to the cytoplasmic face of inside-out membrane vesicles (IOV). The labeling strategy relied on the relative impermeability of MPB to the inner membrane of E.coli (Wada et al., 1999) and the full membrane impermeability of AMS (Long et al., 1998). MPB presumably readily passes through the pores of the outer membrane. Lack of reactivity with cysteines, either within the bilayer or on the interior surface of cells or IOV, was verified by first blocking water-accessible cysteines with membrane-impermeable AMS prior to addition of MPB. Cysteines protected from reactivity with MPB, from the outside of either whole cells or IOV, were exposed by permeabilization of the bilayer with toluene.
Leader peptidase (Lep) has previously been shown to have the same orientation in the inner membrane of E.coli in both PE-containing and PE-lacking cells (Rietveld et al., 1995). Under our conditions, Lep in right-side-out membrane vesicles and IOV also had the same orientation in both cell types, and the IOV were sealed. The conditions for the sidedness-dependent modification of cysteines by MPB were established using whole cells and IOV containing overexpressed Lep (see Supplementary figure 1, available at The EMBO Journal Online).
Orientation of LacY assembled in PE-containing and PE-lacking cells
Previous results indicated a structural rearrangement in the region of the P7 domain of LacY, resulting in the loss of detection by a conformation-specific mAb (Bogdanov and Dowhan, 1999) whose epitope lies within this domain (Sun et al., 1996). To assess the effect of membrane phospholipid composition on the topological organization of LacY, single Cys replacement derivatives (Table I; Figure 1) of a Cys-less derivative of LacY were expressed from plasmids in a PE-containing (containing pDD72GM) or PE-lacking (lacking pDD72GM) strain (AL95) of E.coli that was null in the chromosomal copies of lacY and pssA. The replacements were in putative extramembrane domains connecting TMs, predicted by LacY–PhoA fusion analysis (Calamia and Manoil, 1990) and refined analyses of the whole protein (Venkatesan et al., 2000a,b,c). These derivatives display ≥60% of the normal active transport function of LacY (Frillingos et al., 1998). Control western blot analysis using LacY-specific antibody showed that all these derivatives were present in the membrane fraction at nearly wild-type levels of LacY, and that samples had similar amounts of LacY (see bottom panel of Figure 2D). No labeling of a species of the molecular weight of LacY was detected when a ΔlacY strain expressing Cys-less LacY was probed (Supplementary figure 2). The results presented below are representative of experiments performed twice or more.
Table I. Strains and plasmids used in this study.
| Strains or plasmids | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| W3899 | pssA+ | CGSCa |
| AD90 | pss93::kanR (derivative of W3899) | DeChavigny et al. (1991) |
| AD93 | pss93::kanR recA srl::Tn10 (derivative of AD90) | DeChavigny et al. (1991) |
| AA9256 | pss93::kanR ParaB-pssA+ ΔaraBA (derivative of W3899) | Supplementary data |
| AAL9256 | pss93::kanR ParaB-pssA+ ΔaraBA lacY::Tn9 (derivative of W3899) | Supplementary data |
| AL95 | pss93::kanR lacY::Tn9 (derivative of W3899) | Supplementary data |
| Plasmids | ||
| pAH-PSS | ′araC ParaB-pssA+ ′araD′ oriRKγ tetR ampR | Supplementary data |
| pDD72 | pssA+ camR (temperature sensitive for replication) | DeChavigny et al. (1991) |
| pDD72GM | pssA+ genR camS (temperature sensitive for replication) | This work |
| pT7-5/lacY | OPlac-lacY ampR | Bibi and Kaback (1990) |
| pLacY-N | ampR Cys-less lacY with indicated amino acid (N) replaced byCys, i.e. pLacY-G13 has a G13C replacement | Frillingos et al. (1998) |
aColi Genetic Stock Center.
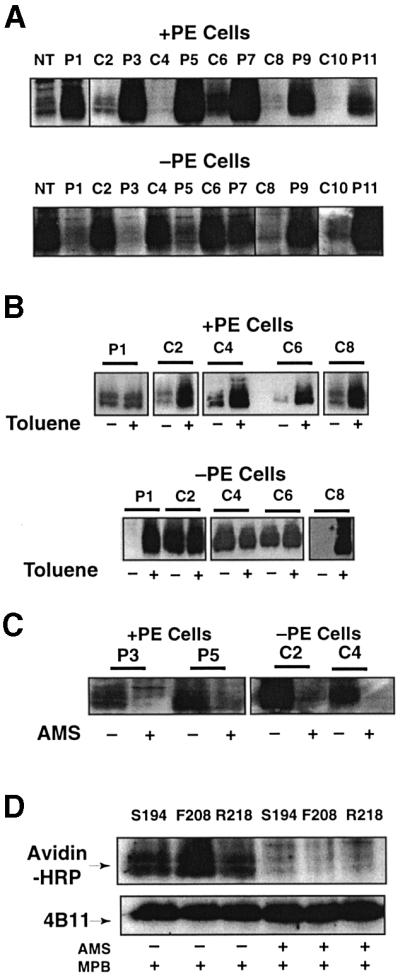
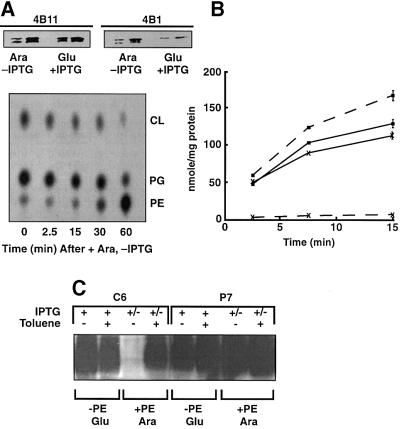
Fig. 2. Determination of LacY topology in PE-containing and PE- lacking cells. Strain AL95 (null in chromosomal pssA and lacY) either with (+PE) or without (–PE) plasmid pDD72GM was used as the host for single Cys replacements of LacY in each P and C domain, as indicated in Figure 1. Unless otherwise noted, plasmid pLacY-F208 was used for analysis of domain C6. The data within each box come from the same autoradiogram, with lines to indicate splicing between lanes. (A) After growth and induction of LacY, whole cells were treated with MBP and the membranes subjected to immunoprecipitation, SDS–PAGE and western blotting using Avidin–HRP to detect biotin linked via the single Cys in each LacY derivative. (B) Where indicated, whole cells were treated with toluene prior to reaction with MBP followed by further analysis as in (A). (C) Cells expressing the indicated Cys derivatives were treated with AMS where indicated prior to treatment with MBP, and then analyzed as in (A). (D) Plasmids expressing single Cys derivatives at the indicated positions in domain C6 were expressed in PE-lacking cells. As indicated, some of the samples were treated with AMS prior to treatment with MPB, followed by analysis as described in (A). LacY derivatives were first visualized using Avidin–HRP (upper panel) followed by stripping of the solid support and visualization with mAb4B11 (lower panel) to quantify levels of total LacY.
The predicted biotinylation patterns (Figure 2A, upper panel) were observed for single Cys derivatives of LacY expressed and probed in PE-containing cells, i.e. all derivatives with single cysteines in the periplasmic (P) domains were labeled, while those with single cysteines in the cytoplasmic (C) domains and the N-terminus were protected from labeling. Unless noted otherwise, all results for C6 were with the F208 replacement derivative. When cells were permeabilized with toluene prior to MPB treatment, a small amount of additional biotinylation occurred for the P1 domain, but extensive biotinylation of C domains occurred (Figure 2B, upper panel). These results are in agreement with the putative location of single cysteines in the current topology map of LacY (Figure 1).
The results with the same single Cys derivatives probed in PE-lacking cells were dramatically different. The N-terminal, C2, C4 and C6 domains were all accessible to MPB from the outside of whole cells while the P1, P3, and P5 domains were all protected from reaction with MPB (Figure 2A, lower panel). The remainder of the domains from P7 to P11 behaved the same as in PE-containing cells. Consistent with these results, P1 and C8 were rendered accessible to MPB after toluene treatment, while there was no increase in the accessibility of C2, C4 or C6 (Figure 2B, lower panel). Cysteines accessible to MPB in PE-containing and PE-lacking whole cells were blocked from reaction with MPB if cells were first treated with AMS (Figure 2C). The same was true for three different single Cys derivatives, all in the C6 domain, that were expressed independently and accessible in PE-lacking cells (Figure 2D). This further establishes that MPB did not react with cysteines either sequestered in the membrane bilayer or in the lumen.
To substantiate further the misorganization of LacY in PE-lacking cells, single Cys derivatives in IOV isolated from both cell types were probed. The labeling patterns for IOV without (Figure 3A) and with (Figure 3B) toluene treatment was a mirror image of the labeling pattern observed in whole cells. As with whole cells, pre-treatment with AMS blocked reaction of accessible domains with MPB (Figure 3C). The complementary and opposite labeling pattern observed with IOV strongly supports the major topological differences observed between PE-containing and PE-lacking cells.
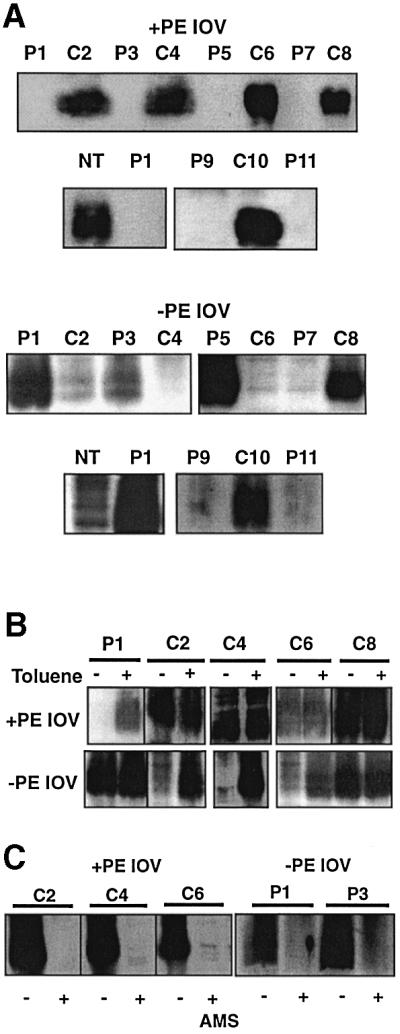
Fig. 3. Determination of LacY topology in IOV prepared from PE-containing and PE-lacking cells. The same plasmid and cell combinations were used as described in Figure 2. IOV were prepared as described in Materials and methods from cells expressing the indicated Cys replacements, followed by treatment of the vesicles with MBP alone (A), with (+) or without (–) toluene prior to MPB treatment (B), or with (+) or without (–) AMS prior to MPB treatment (C).
For both Lep (Supplementary figure 1) and LacY, putative inaccessible cysteines showed some labeling (ranging from 5 to 15%) even in the absence of toluene and there was some increase in labeling of putative accessible cysteines after toluene treatment (5–15%). This ‘leak-through’ labeling varied between experiments (compare C4, C6 and C8 for PE-containing cells in Figure 2A and B, and compare C2 and C6 for PE-lacking IOV in Figure 3A and B) and was observed for both PE-containing and PE-lacking cells. This labeling was probably due to partial penetration of the inner membrane by MPB. The increase in labeling of accessible cysteines after toluene treatment (P1 for PE-containing cells and C2 for PE-lacking cells in Figure 2B) was variable between experiments. However, in all cases where cysteines were not labeled or poorly labeled, addition of toluene dramatically increased labeling. Where labeling was strong, addition of toluene only moderately increased labeling. The latter was probably due to toluene-induced local changes in accessibility of exposed cysteines.
The C-terminus of LacY remains in the correct orientation, regardless of the presence or absence of PE. A derivative of LacY was used with a Factor Xa protease site at the N-terminus of an 100-amino acid biotinylation domain attached as an extension to the C-terminus of LacY. This derivative was nearly completely degraded by Factor Xa protease with the same efficiency for IOV derived from either PE-containing (Zen et al., 1995) or PE-lacking cells (Supplementary figure 3).
Several conclusions follow from these findings. From domain P7 to the C-terminus of LacY, the topological organization of LacY is the same when expressed in either PE-containing or PE-lacking cells. However, expression of LacY in PE-lacking cells results in global perturbations in which the N-terminus through domain C6 adopts an inverted topology, where normally non-translocated hydrophilic loops connecting TMs are translocated while normally translocated loops are not. Within the limits of the leak-through reactivity noted above, a uniform population of LacY is generated by assembly either in PE-containing or PE-lacking cells.
A reversible topological switch triggered by a change in phospholipid composition
Can the misorganization of LacY be corrected after assembly by the addition of PE? In vitro translation and assembly experiments indicated that LacY assembled in IOV lacking PE and lacking the structural determinants within domain P7 recognized by mAb4B1 could regain these structural determinants by post-assembly synthesis of PE within the vesicles. LacY is also recognized by mAb4B11, independent of PE, provided that LacY is integrated into the membrane (Bogdanov and Dowhan, 1998). This is presumably the result of the now established proper alignment, independent of PE, of the discontinuous epitope contributed by domains C8 and C10.
In a strain with the chromosomal pssA gene under tight regulation of the araB promoter, the synthesis of PE can be induced by growth in the presence of arabinose or repressed by growth on glucose. The chromosomal or plasmid-borne copy of the lacY gene under control of its own promoter can be induced by the addition of isopropyl 1-thio-β-d-galactopyranoside (IPTG). Cells were grown first on glucose and IPTG in order to allow synthesis and membrane assembly of LacY in the near absence of PE. Then glucose and IPTG were removed, and cells were grown on arabinose to allow new synthesis of PE in the absence of new LacY synthesis. Cells were isolated and assayed for transport function and Cys accessibility, or membranes were isolated and subjected to western blot analysis with conformation-specific mAbs. Parallel cultures either expressing wild-type LacY or only a single Cys derivative were grown with 32PO4 to determine phospholipid composition at the times indicated.
The results shown in Figure 4A were from cells carrying both a chromosomal and plasmid-borne copy of wild-type lacY. LacY overexpressed from the plasmid gene predominates. The level of PE is <3% of total phospholipid in cells grown in glucose (Figure 4A, lower panel, time 0). As demonstrated previously (Bogdanov et al., 1996), LacY assembled in the absence of PE (Figure 4A, upper panel, Glu +IPTG) was poorly recognized by mAb4B1 (conformation dependent), indicating a lack of proper conformation of the P7 domain. Recognition by mAb4B11 (membrane insertion dependent but PE independent) indicated the presence of ample LacY integrated into the membrane (Bogdanov and Dowhan, 1998). Switching to growth on arabinose in the absence of IPTG returned phospholipid composition to normal after 60 min (Figure 4A, lower panel). With restored PE levels, recognition of previously synthesized LacY by mAb4B1 was restored (Ara, –IPTG), indicating a regain of native conformation of the P7 domain. The intensity of the signal with mAb4B11 was reduced by ∼50%, with the same total membrane protein load as the sample from glucose grown cells. This is consistent with no new synthesis of LacY after the switch to arabinose but with a dilution of existing LacY with continued cell growth.
Fig. 4. Reversibility of LacY topology. In (A), strain AA9256/pT7-5/lacY (chromosomal pssA expression under arabinose regulation and lacY under IPTG regulation) was used. In (B) strain AAL9256/pLacY-F208 (loop C6) was used, and in (C) strain AAL9256/pLacY-F208 (loop C6) or AAL9256/pLacY-F250 (loop P7) was used (chromosomal pssA expression under arabinose regulation, chromosomal ΔlacY, and plasmid lacY under IPTG regulation with single Cys derivatives in either domain C6 or P7). Cells were first grown to an OD600 of 0.2 in the presence of glucose (Glu) and IPTG. Cells were washed twice by centrifugation to remove glucose and IPTG and then grown for 60 min in medium containing arabinose (Ara). (A) In the upper panel cell membranes were isolated after growth in either Glu plus IPTG or Ara minus IPTG (after first being grown in Glu plus IPTG), and subjected to SDS–PAGE and western blotting using the monoclonal antibodies indicated. Each pair of results represents 12.5 µg (left) or 25 µg (right) of membrane protein. In the lower panel, parallel cultures were first radiolabeled to constant specific activity with and then maintained in 32PO4 during growth, first in Glu plus IPTG followed by Ara minus IPTG. Cells were analyzed for their phospholipid (PG, phosphatidylglycerol; CL, cardiolipin) content (DeChavigny et al., 1991) at the times indicated after switching from Glu (time 0) to Ara. (B) Cells were grown first in Glu plus IPTG (dashed lines) followed by Ara minus IPTG (solid lines) as described above. LacY transport function using either uptake of lactose (filled squares) or TMG (crosses) to measure facilitated or active transport, respectively, was carried out after Glu and after Ara growth. Error bars for duplicate samples are indicated where significant. (C) Cells were analyzed either after growth in the presence of IPTG (+) plus glucose (–PE Glu) or after removing IPTG (+/–) and glucose, and grown for 60 min in the presence of arabinose (+PE Ara). Where indicted, cells were treated with toluene prior to treatment with MBP followed by analysis by western blotting as described in Figure 2. Parallel cultures were analyzed for phospholipid content and transport function.
LacY expressed from pLacY-F208 (as well as from pLacY-F250 and pT7-5/lacY; not shown) and assembled in PE-lacking ΔlacY cells (Figure 4B) carried out facilitated transport of lactose (uptake and hydrolysis by β-galactosidase) but not active transport of the non-hydrolyzable substrate analog methyl-thio-β-d-galactopyranoside (TMG). After recovery of PE levels to normal after arabinose growth in the absence of IPTG, active transport was restored and the rate of facilitated transport per milligram of protein was reduced again, consistent with no new LacY synthesis. Only the basal level of LacY active transport (as in Figure 4B) was detected in cells grown in the presence of IPTG and glucose, or in the absence of IPTG with growth in either glucose or arabinose (not shown).
In Figure 4C, strains with a chromosomal ΔlacY were used so there would be no interference with the analysis of plasmid-encoded single Cys derivatives of LacY. Treatment of intact cells expressing single Cys derivatives of LacY (+IPTG) with MPB showed that after growth on glucose (–PE, Glu), the C6 and P7 domains were biotinylated with and without toluene treatment, consistent with their periplasmic orientation. After removal of IPTG (+/–IPTG) and growth on arabinose (+PE, Ara), domain C6 was no longer accessible to MPB unless cells were first treated with toluene. Domain P7 remained accessible with and without toluene. The lack of increased reactivity of accessible cysteines after toluene treatment and the dependence of the accessibility of the C6 domain on toluene after return of PE to normal levels strongly supports a ‘sliding back’ of the C6 domain to the cytoplasmic side of the membrane.
In order to establish that the changes in LacY biotinylation patterns observed after switching growth from glucose to arabinose are not due to instability of LacY or read-through expression of ‘new’ LacY in the absence of IPTG, the radiolabeling experiments described in Figure 5 were performed under the same growth conditions as described in Figure 4C. Cell lysis and isolation of membranes was carried out using NaOH treatment to obtain detectable levels of radiolabeled material from cells labeled in rich medium. LacY expressed and labeled during growth in the presence of glucose and IPTG (Figure 5A) is stable during a 60-min chase of radiolabel (by direct counts and exposure intensity) after switching to growth in the presence of arabinose and in the absence of IPTG. Therefore, the loss of MPB labeling of domain C6 after inducing the synthesis of PE cannot be due to degradation of LacY made in the absence of PE. Treatment with NaOH resulted in a significant amount of LacY dimer. In vitro biotinylated LacY is also displayed as both a monomer and dimer (Figure 5A, lane a) after NaOH treatment, as opposed to only a monomer (lane b), when using the cell lysis method outlined in Materials and methods. Furthermore, there is no detectable LacY expressed during growth in the presence of arabinose without IPTG (Figure 5B). Therefore, the toluene-dependent MPB labeling of domain C6 after induction of PE synthesis cannot be due to newly synthesized LacY.
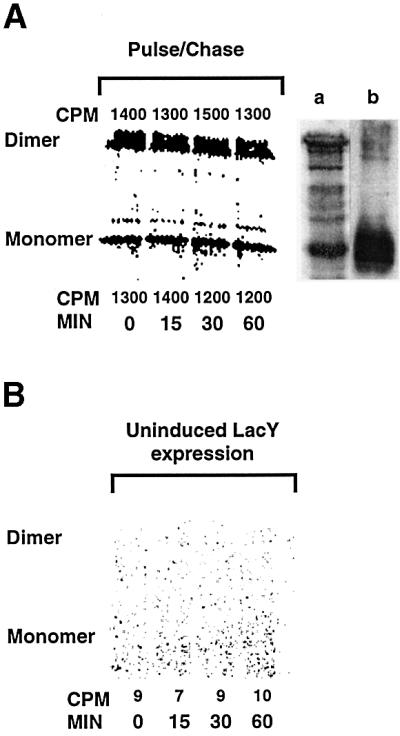
Fig. 5. Stability and read-through expression of LacY. (A) Growth conditions for strain AAL9256/pLac-F208 (domain C6) and induction of LacY and PE synthesis were exactly as described in Figure 4C, except as follows. During growth in the presence of IPTG and glucose (minus PE plus LacY), the media was supplemented with 40 µCi/ml of [35S]methionine/cysteine during growth for 60 min. After removal of glucose, IPTG and radiolabel, cells were resuspended in LB medium containing unlabeled methionine/cysteine (0.05% each) and arabinose to induce PE synthesis (time 0) in the absence of LacY induction. At the times (min) indicated, equal aliquots were removed, cells were harvested by centrifugation, the pellets were rapidly frozen then thawed, resuspended in 250 µl of buffer A and lysed by addition of an equal volume of 0.4 M NaOH followed by incubation for 20 min on ice. The membranes were harvested by centrifugation, washed once with 4 M KCl, and washed once with Tris–HCl buffer pH 8.1. The pellets were solubilized at 37°C and subjected to immunoprecipitation as described in Materials and methods, and subjected to SDS–PAGE followed by imaging and quantification with a Beta Imager from Packard Ltd. Permease remaining at each time point was expressed as counts per minute (CPM), measured at the positions corresponding to either LacY monomers or dimers. Samples grown without radiolabel in glucose plus IPTG and labeled with MPB were prepared for immunoprecipitation and western blotting either by NaOH treatment as above (lane a) or as described in Materials and methods (lane b). (B) Cells were grown as described above, except radiolabel was added during growth in the presence of arabinose and in the absence of IPTG and methionine/ cysteine. Aliquots were removed at the times indicated after the addition of radiolabel and subjected to analysis of radiolabeled protein as above.
Discussion
We established that PE plays an active role in determining the topological organization of a polytopic membrane protein, either during initial membrane assembly or dynamically after membrane insertion has occurred. Topological differences within LacY dependent on PE were assessed by differences in accessibility using membrane impermeable sulfhydryl reagents to single Cys replacements in the extra-membrane domains connecting TMs. The advantage of this technique is that modification is restricted to a single amino acid residue that is less likely to disturb the overall structure (van Geest and Lolkema, 2000). Conclusions are based on the use of complementary approaches comparing accessibility in PE-containing versus PE-lacking membranes, whole cells versus IOV, and sealed membranes versus toluene-permeabilized membranes. The internally consistent and complementary results demonstrate that the sulfhydryl reagents employed were only slightly membrane permeable.
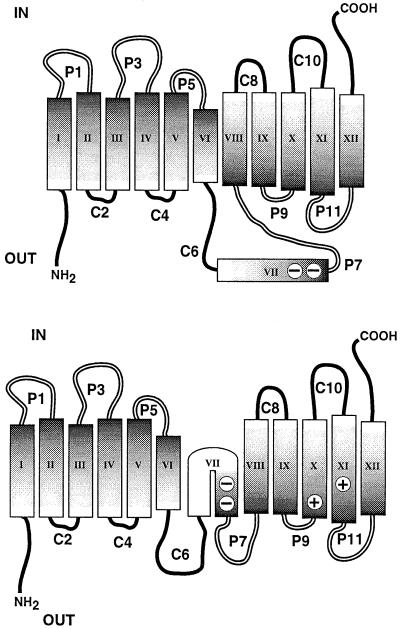
The molecular basis for the changes in LacY structure and function brought about by assembly in PE-lacking membranes is a surprising change in its topological organization (Figure 6). Our data strongly support the complete inversion of the first six TMs, with retention of normal topology of the last five TMs. Our results cannot distinguish between a looping out of TM VII into the periplasm (upper panel) and a ‘U’-shaped mini-loop partially inserted into the periplasmic side of the membrane (lower panel). The low hydrophobicity of TM VII in combination with the flanking C6 and P7 domains might stabilize this large domain outside of the membrane bilayer. On the other hand, TM VII contains Asp237 and Asp240, which are stabilized in the membrane via salt bridges with Lys358 (TM XI) and Lys319 (TM X), respectively (Kaback and Wu, 1999). The lack of native structure of the P7 domain (Bogdanov et al., 1996) is consistent with a change in organization of LacY in this region. Such misorganization of LacY does not preclude partial transport function since a derivative lacking the N-terminal half of LacY through the middle of C6 still carries out facilitated transport (Wu et al., 1996).
Fig. 6. Model for LacY topological organization in PE-lacking cells. Both panels indicate the cytoplasmic face (IN) and periplasmic face (OUT) of LacY, and inversion of the N-terminal six TMs and associated extramembrane loops. The upper panel shows TM VII, with its two acidic amino acid residues (–) looped out into the periplasmic space. The lower panel depicts TM VII as a ‘U’-shaped mini-loop in the membrane bilayer, stabilized by salt bridges to the indicated basic amino acid residues (+).
The most surprising result is the post-insertional triggering by PE of changes resulting in the proper conformation of domain P7, the return of normal topological organization of domain C6, and the restoration of a catalytically competent transporter. Although only the C6 and P7 domains were probed in these experiments, active transport function of LacY requires interaction of TM II with TM VII and TM XI (Wu and Kaback, 1997), suggesting additional TM reorientation. Previous experiments demonstrated that regain of recognition of LacY by mAb4B1 (regain of P7 structure) does occur by post-assembly addition of PE in vitro (Bogdanov and Dowhan, 1998).
What could be the molecular basis for the drastic change in the topological organization of LacY in the absence of PE? Phospholipids might exert their effect on membrane protein topology directly by interacting with newly synthesized proteins to modify or determine their topogenic signals. Alternatively, membrane phospholipid composition or specific phospholipids may directly affect the specificity or function of the translocon/insertase complex, thereby changing how TMs are organized for a subset of proteins. However, refolding and restoration of the antigenicity of domain P7 of LacY in vitro, solely dependent on PE (Bogdanov et al., 1999), favors LacY–lipid interactions as the primary determinant of topological organization.
LacY appears to be inserted into the membrane cotranslationally (Stochaj and Ehring, 1987), utilizing the signal recognition particle and FtsY (Seluanov and Bibi, 1997). Assembly of functional LacY has been reported both independently of SecAY (Yamato, 1992) and dependent on SecY (Ito and Akiyama, 1991). However, most of the evidence for SecY involvement in polytopic membrane protein assembly is based on using chimeric fusions with reporter proteins that require SecY for membrane translocation (Prinz et al., 1998), and therefore may not reflect the mechanism by which native proteins are assembled (Pugsley, 1993).
Since PE-lacking cells are viable, it is unlikely that the lack of PE can have a broad-ranging effect on the insertion machinery, but a subset of proteins may be affected. The essential membrane protein Lep, which appears to utilize SecAY and YidC (Houben et al., 2000), is functional and properly oriented in PE-lacking cells. SecA is not reduced in PE-lacking cells (Rietveld et al., 1995); however, some mutations in SecY result in topological changes for chimeric derivatives of at least one polytopic membrane protein (Prinz et al., 1998). Although PE is required for FtsY membrane association in vitro, FtsY-dependent membrane insertion of AcrB is normal in PE-lacking cells (Millman et al., 2001).
There is ample evidence to indicate that the two halves of LacY behave as independent domains during membrane insertion and therefore are able to respond differently to topological signals and determinants, consistent with our results. The N-terminal half (through domain C6) of LacY when expressed separately is membrane associated but unstable. However, coexpression of the two halves of LacY as independent proteins results in a stable active permease (Bibi and Kaback, 1990). The C-terminal half when expressed by itself is inserted and stable in the membrane, and carries out facilitated transport of substrate (Wu et al., 1996). The length of domain C6 may play a role during assembly of LacY by inducing a pause in TM insertion, possibly allowing the first six TMs to assemble before insertion of the remaining TMs (Weinglass and Kaback, 2000).
The cytoplasmic domains of LacY obey the ‘positive inside’ rule (von Heijne, 1992). Orientation of such positively charged domains to the cytoplasmic side of the membrane is favored by the negative-inside electrochemical potential across the cytoplasmic membrane (Andersson and von Heijne, 1994) and their interaction with the head groups of anionic phospholipids (van Klompenburg et al., 1997), neither of which are limiting in PE-lacking cells (Bogdanov and Dowhan, 1995). However, domains C2 and C4 (N-terminal half) are very short, and acidic amino acids are positioned near the membrane–cytoplasmic interface of domains C2 (Asp68), C4 (Glu126) and C6 (Glu190), which are conserved among sugar permeases of Gram-negative bacteria (Varela and Wilson, 1996). Negatively charged amino acids, particularly near this interface (Rutz et al., 1999), favor translocation of hydrophilic loops to the periplasmic side of the membrane. The cytoplasmic loops in the N-terminal half may be in a metastable or ‘frustrated’ state due to the opposing signals of positively and negatively charged amino acids making them more prone to respond to changes in the surface charge of the membrane. The lack of PE might increase the negative charge density at the membrane surface, making membrane translocation favorable (Krishtalik and Cramer, 1995). Increasing the anionic lipid content in a liposome system favors the translocation of slightly positively charged domains in a Sec-independent manner, as opposed to the positive-inside rule (Ridder et al., 2001).
It is unlikely that the reversibility in topology is independent of the protein assembly machinery. Given the stability and partial function of LacY assembled in the absence PE, the protein appears to be in a compact structure either trapped in a metastable conformation or at an energy minimum with respect to its lipid environment. Numerous studies indicate that LacY is a flexible and highly mobile structure (Prive and Kaback, 1996; Venkatesan et al., 2000a,b,c). Exposure to PE may destabilize LacY, resulting in partial unfolding of the protein and re-entry into the translocon/insertase machine for reorientation, but now following the new orientation rules dictated by the presence of PE. Therefore, the assembly machinery may be responsible for assuring native protein structure via a post-assembly proofreading process.
Several proteins displaying multiple structural arrangements depend on interaction with lipids. Peptide-induced membrane fusion in vitro is regulated by trace amounts of lysophospholipids in target membranes that induce the conversion of a fusion-permissive α-helical conformation to a non-fusogenic β-sheet (Pecheur et al., 2000); therefore local changes in lipid composition can affect protein structure. The L envelop protein of hepatitis B virus exists in two topological forms (Lambert and Prange, 2001). Ductin exists as a subunit of the vacuolar H+-ATPase and in an opposite orientation in microsomes as a component of the connexon channel of gap junctions (Dunlop et al., 1995). A microsomal epoxide hydroxylase is found with a different topology in the endoplasmic reticulum than in the sinusoidal plasma membrane, where it mediates bile acid transport (Zhu et al., 1999). Do these differences originate during membrane insertion, or are they induced by changes in lipid composition as proteins move through the different organelles to their final destination? An outer envelope protein of chloroplasts (OEP7) orients with native topology in liposomes reflecting in vivo lipid composition, but with an opposite topology in liposomes of non-native composition (Schleiff et al., 2001). Therefore, protein topology can be determined by both the protein sequence and membrane lipid composition.
The unique and novel contribution made by the studies reported here is that the evolving model for integral membrane protein assembly must take into account both the specific membrane lipid composition in establishing membrane protein topology and the potential for post-assembly TM reorganization in response to the lipid environment. By utilizing mutants lacking PE we were able to identify steps in membrane protein assembly involving phospholipids that could not be recognized or studied in wild-type cells.
Materials and methods
Materials
Chemicals and enzymes were purchased from Sigma Chemical Corp. Radiolabeled material and the peroxidase-labeled antibody enhanced chemiluminescence detection (ECL) kit came from Amersham Corp. MPB and AMS were purchased from Molecular Probes, Inc. Polyclonal antibody (pAb) directed against the C-terminus of LacY, mAb4B1 directed against the properly folded P7 domain of LacY (Sun et al., 1996), and mAb4B11 directed against a discontinuous epitope composed of the C8 and C10 domains of LacY (Sun et al., 1996) were obtained from H.R.Kaback (University of California, Los Angeles). Avidin–HRP was from Pierce. Pansorbin cells were from Calbiochem, and Lubrol (type-PX) from Nacalai Tesque, Inc. (Kyoto, Japan). Nitrocellulose sheets for immunoblotting were purchased from Schlicher and Schuell.
Strains and plasmids
The source and relevant characteristics of E.coli strains and plasmids used are summarized in Table I. A series of plasmids encoding derivatives of LacY containing single Cys replacements in a Cys-less LacY [see Frillingos et al. (1998) for description and characterization of these plasmids] at the indicated positions of wild-type LacY were provided by H.R.Kaback. The original nomenclature for these plasmids was changed, e.g. from ‘pT7-5/C-less lacY/G13C’ to ‘pLacY-G13’.
Cells were grown in Luria–Bertani (LB)-rich medium and supplemented with ampicillin (100 µg/ml), kanamycin (40 µg/ml), tetracycline (10 µg/ml), chloramphenicol (12.5 µg/ml) or gentamicin (15 µg/ml) as required for selection. Strains carrying the pss93::kan null allele require either a functional plasmid-borne copy of the pssA gene (plasmid pDD72 or pDD72GM) or growth media containing 50 mM MgCl2 for viability (DeChavigny et al., 1991). The wild-type (PE-containing) control strain was a pss93::kan null-allele-containing strain (PE-deficient) carrying either pDD72 or pDD72GM. All strains (mutant and wild type for phospholipid composition) were grown at 30°C in 50 mM MgCl2. Expression of the lacY gene, either on plasmids or from the chromosome, was carried out in strains grown for at least two generations after induction with 1 mM IPTG at an OD600 of ∼0.15. Expression of the pssA under control of ParaB was either induced by growth in the presence of 0.25% arabinose or repressed by growth in the presence of 2% glucose; in order to maintain control of expression under ParaB regulation, cells were maintained in exponential growth.
Preparation and protease treatment of membrane vesicles
IOV were prepared as described previously (Bogdanov and Dowhan, 1995) by rupture of cells using a French press at 560 kg/cm2 (8000 p.s.i.). Vesicles were concentrated by centrifugation, resuspended in ice-cold 100 mM HEPES buffer (pH 8.0) containing 250 mM sucrose, 25 mM MgCl2 and 1 mM dithiothreitol (DTT) at a protein concentration of 5–10 mg/ml, and stored at –80°C. DTT was omitted in IOV preparations used for site-directed thiol-labeling experiments.
Chemical labeling of Cys residues
A 100 ml culture of cells expressing a single Cys derivative of LacY was grown in LB medium plus Mg2+ at 30°C to an OD600 of 0.5–0.7, resuspended after harvesting to an OD600 of 50 in buffer A (100 mM K–HEPES, 250 mM sucrose, 25 mM MgCl2, 0.1 mM KCl pH 7.5), and divided into several 200 µl aliquots. Samples were brought to 5 mM in AMS and incubated for 30 min at 25°C to block water-accessible Cys residues from the outside of cells. AMS was removed by two cycles of centrifugation and resuspension in buffer A. Cells, either pre-treated or not with AMS, were biotinylated by adding MPB [10–50 mM, freshly dissolved in dimethyl sulfoxide (DMSO)] to 100 µM followed by incubation for 5 min at 25°C; final DMSO concentration never exceeded 0.5%. The reaction was quenched by addition of βME to 20 mM, followed by two cycles of centrifugation and resuspension in buffer A containing 20 mM βME. To expose internal Cys residues to external solvent, cell suspensions were vortexed vigorously with 2 µl of toluene for 15 s and subjected to labeling as described above. Finally, labeled cells were resuspended in buffer A containing 20 mM βME, then solubilized and immunoprecipitated as described below.
IOV (100 µg of protein in 100 µl of buffer A) were first incubated in either the presence or absence of 0.5 mM AMS for 30 min at 25°C prior to biotinylation by incubation in 20 µM MPB for 5 min at 25°C, followed by quenching with 20 mM βME. AMS was removed before reaction with MPB or unreacted MPB was removed at end of labeling by passing the reaction mixture by low speed centrifugation through a mini Sephadex G-50 column prepared in buffer A either without or with 20 mM βME, respectively. The labeled IOV were solubilized and immunoprecipitated as described below.
Immunoprecipitation and western blot analysis
Labeling of Cys residues by MPB was measured after detergent solubilization, immunoprecipitation, SDS–PAGE and western blotting using Avidin-linked HRP as described below. After MPB labeling, cells or vesicles were solubilized by addition of an equal volume of 50 mM Tris–HCl buffer pH 8.1, 2% SDS and 1 mM EDTA followed by vigorous vortexing for 15 min at room temperature, incubation at 37°C for 15 min, and an additional 15 min vortexing at room temperature. Samples (200 µl) were diluted with 300 µl of cold 50 mM Tris–HCl buffer pH 8.1 containing 0.15 M NaCl, 2% Lubrol-PX, 0.4% SDS and 1 mM EDTA (buffer IP1), and immunoprecipitated with anti-LacY pAb overnight at 4°C. The antibody complex was isolated by the addition of 50 µl of a suspension of formalin-treated Staphylococcus aureas cells (Pansorbin) reconstituted in buffer IP1 according to the supplier’s instructions. The samples were further incubated at 4°C with mixing every 5 min for 1 h. After centrifugation the pellet was washed once with buffer IP1, once with 0.75 ml of 50 mM Tris–HCl pH 8.1, 1 M NaCl, 0.1% Lubrol-PX, 0.2% SDS, and once with 10 mM Tris–HCl pH 8.1. The final precipitates were solubilized by vortexing in 50 µl of SDS sample buffer (10 mM Tris–HCl pH 6.8, 5.6% SDS, 200 mM DTT, 10% glycerol, 0.01% bromophenol blue) as described above. Samples were centrifuged and the solubilized proteins were subjected to PAGE (12.5% gels).
The samples were transferred from SDS–polyacrylamide gels to nitrocellulose membranes (0.2 µm) as described previously (Bogdanov et al., 1996). Avidin–HRP (1:50 000 dilution of 2 mg/ml stock solution) using ECL reagents was used to visualize biotinylated proteins according to the manufacturer’s instructions. To ensure the presence of equal amounts of antigen in each sample, blots were stripped (Amersham suggested following the protocol accompanying the ECL kit) and reprobed with the mAb4B11 against LacY followed by HRP-conjugated secondary antibody, and developed using an ECL detection method (Bogdanov et al., 1996). To determine LacY levels in cells grown first on glucose with IPTG, followed by arabinose minus IPTG and arabinose minus glucose, cell membranes were isolated, subjected to SDS–PAGE and analyzed by western blotting using either mAb4B1 or mAb4B11 as described previously (Bogdanov and Dowhan, 1998). Cells were labeled with 32PO4 and the phospholipid composition was analyzed (DeChavigny et al., 1991) using chloroform/methanol/acetic acid (65/25/8, v/v) as chromatography solvent.
Other methods
Transport of [3H]TMG (0.5 µCi/ml) and [14C]lactose (0.5 µCi/ml) at final concentrations of 0.1 and 0.4 mM, respectively, was measured in intact E.coli cells harboring the indicated plasmids as described previously (Bogdanov and Dowhan, 1995).
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr H.R.Kaback for providing the collection of LacY derivatives and LacY antibodies, without which this work would not have been possible. Dr G.Kramer suggested using a high SDS/non-ionic detergent ratio during immunoprecipitation that was instrumental in designing an immunoprecipitation protocol for LacY. This work was supported by grant GM-20478 from the National Institutes of Health (to W.D.).
References
- Andersson H. and von Heijne,G. (1994) Membrane protein topology: effects of ΔµH+ on the translocation of charged residues explain the ‘positive inside’ rule. EMBO J., 13, 2267–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H.D. (2000) The biogenesis and assembly of bacterial membrane proteins. Curr. Opin. Microbiol., 3, 203–209. [DOI] [PubMed] [Google Scholar]
- Bibi E. and Kaback,H.R. (1990) In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc. Natl Acad. Sci. USA, 87, 4325–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M. and Dowhan,W. (1995) Phosphatidylethanolamine is required for in vivo function of the membrane-associated lactose permease of Escherichia coli. J. Biol. Chem., 270, 732–739. [DOI] [PubMed] [Google Scholar]
- Bogdanov M. and Dowhan,W. (1998) Phospholipid-assisted protein folding: phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J., 17, 5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov M. and Dowhan,W. (1999) Lipid-assisted protein folding. J. Biol. Chem., 274, 36827–36830. [DOI] [PubMed] [Google Scholar]
- Bogdanov M., Sun,J., Kaback,H.R. and Dowhan,W. (1996) A phospholipid acts as a chaperone in assembly of a membrane transport protein. J. Biol. Chem., 271, 11615–11618. [DOI] [PubMed] [Google Scholar]
- Bogdanov M., Umeda,M. and Dowhan,W. (1999) Phospholipid-assisted refolding of an integral membrane protein. Minimum structural features for phosphatidylethanolamine to act as a molecular chaperone. J. Biol. Chem., 274, 12339–12345. [DOI] [PubMed] [Google Scholar]
- Calamia J. and Manoil,C. (1990) Lac permease of Escherichia coli: Topolology and sequence elements promoting membrane insertion. Proc. Natl Acad. Sci. USA, 87, 4937–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R.E., Chen,M., Jiang,F. and Samuelson,J.C. (2000) Understanding the insertion of transporters and other membrane proteins. Curr. Opin. Cell Biol., 12, 435–442. [DOI] [PubMed] [Google Scholar]
- DeChavigny A., Heacock,P.N. and Dowhan,W. (1991) Sequence and inactivation of the pss gene of Escherichia coli: phosphatidylethanolamine may not be essential for cell viability. J. Biol. Chem., 266, 5323–5332. [PubMed] [Google Scholar]
- de Gier J.W., Scotti,P.A., Saaf,A., Valent,Q.A., Kuhn,A., Luirink,J. and von Heijne,G. (1998) Differential use of the signal recognition particle translocase targeting pathway for inner membrane protein assembly in Escherichia coli. Proc. Natl Acad. Sci. USA, 95, 14646–14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J., Jones,P.C. and Finbow,M.E. (1995) Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J., 14, 3609–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov R.G. and Vergoten,G. (1996) Recognition of transmembrane α-helical segments with environmental profiles. Protein Eng., 9, 253–263. [DOI] [PubMed] [Google Scholar]
- Frillingos S., Sahin-Toth,M., Wu,J. and Kaback,H.R. (1998) Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J., 12, 1281–1299. [DOI] [PubMed] [Google Scholar]
- Gafvelin G. and von Heijne,G. (1994) Topological ‘frustration’ in multispanning E. coli inner membrane proteins. Cell, 77, 401–412. [DOI] [PubMed] [Google Scholar]
- Houben E.N., Scotti,P.A., Valent,Q.A., Brunner,J., de Gier,J.L., Oudega,B. and Luirink,J. (2000) Nascent Lep inserts into the Escherichia coli inner membrane in the vicinity of YidC, SecY and SecA. FEBS Lett., 476, 229–233. [DOI] [PubMed] [Google Scholar]
- Ito K. and Akiyama,Y. (1991) In vivo analysis of integration of i proteins in Escherichia coli. Mol. Microbiol., 5, 2243–2253. [DOI] [PubMed] [Google Scholar]
- Kaback H.R. and Wu,J. (1999) What to do while awaiting crystals of a membrane transport protein and thereafter. Acc. Chem. Res., 32, 805–813. [Google Scholar]
- Kaback H.R., Sahin-Toth,M. and Weinglass,A.B. (2001) The kamikaze approach to membrane transport. Nature Rev. Mol. Cell Biol., 2, 610–620. [DOI] [PubMed] [Google Scholar]
- Kim H., Paul,S., Jennity,J. and Inouye,M. (1994) Reversible topology of a bifunctional transmembrane protein depends upon the charge balance around its transmembrane domain. Mol. Microbiol., 11, 819–831. [DOI] [PubMed] [Google Scholar]
- Krishtalik L.I. and Cramer,W.A. (1995) On the physical basis for the cis-positive rule describing protein orientation in biological membranes. FEBS Lett., 369, 140–143. [DOI] [PubMed] [Google Scholar]
- Lambert C. and Prange,R. (2001) Dual topology of the hepatitis B virus large envelope protein: determinants influencing posttranslational translocation. J. Biol. Chem., 276, 22265–22272. [DOI] [PubMed] [Google Scholar]
- Long J.C., Wang,S. and Vik,S.B. (1998) Membrane topology of subunit a of the F1F0 ATP synthase as determined by labeling of unique cysteine residues. J. Biol. Chem., 273, 16235–16240. [DOI] [PubMed] [Google Scholar]
- Millman J.S., Qi,H.-Y., Vulcu,F., Bernstein,H.D. and Andrews,D.W. (2001) FtsY binds to the Escherichia coli inner membrane via interactions with phosphatidylethanolamine and membrane proteins. J. Biol. Chem., 276, 25982–25989. [DOI] [PubMed] [Google Scholar]
- Pecheur E.I., Martin,I., Bienvenue,A., Ruysschaert,J.M. and Hoekstra,D. (2000) Protein-induced fusion can be modulated by target membrane lipids through a structural switch at the level of the fusion peptide. J. Biol. Chem., 275, 3936–3942. [DOI] [PubMed] [Google Scholar]
- Prinz W.A., Boyd,D.H., Ehrmann,M. and Beckwith,J. (1998) The protein translocation apparatus contributes to determining the topology of an integral membrane protein in Escherichia coli. J. Biol. Chem., 273, 8419–8424. [DOI] [PubMed] [Google Scholar]
- Prive G.G. and Kaback,H.R. (1996) Engineering the lac permease for purification and crystallization. J. Bioenerg. Biomembr., 28, 29–34. [PubMed] [Google Scholar]
- Pugsley A.P. (1993) The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev., 57, 50–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder A.N., Kuhn,A., Lillian,A. and de Kruijff,B. (2001) Anionic lipids stimulate Sec-independent insertion of a membrane protein lacking charged amino acids. EMBO rep., 21, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld A.G., Koorengevel,M.C. and de Kruijff,B. (1995) Non-bilayer lipids are required for efficient protein transport across the plasma membrane of Escherichia coli. EMBO J., 14, 5506–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz C., Rosenthal,W. and Schulein,R. (1999) A single negatively charged residue affects the orientation of a membrane protein in the inner membrane of Escherichia coli only when it is located adjacent to a transmembrane domain. J. Biol. Chem., 274, 33757–33763. [DOI] [PubMed] [Google Scholar]
- Saier M.H. et al. (1999) The major facilitator superfamily. J. Mol. Microbiol. Biotechnol., 1, 257–279. [PubMed] [Google Scholar]
- Schleiff E., Tien,R., Salomon,M. and Soll,J. (2001) Lipid composition of outer leaflet of chloroplasts outer envelope determines topology of OEP7. Mol. Biol. Cell, 12, 4090–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A. and Bibi,E. (1997) FtsY, the prokaryotic signal recognition particle receptor homologue, is essential for biogenesis of membrane proteins. J. Biol. Chem., 272, 2053–2055. [DOI] [PubMed] [Google Scholar]
- Stochaj U. and Ehring,R. (1987) The N-terminal region of Escherichia coli lactose permease mediates membrane contact of the nascent polypeptide chain. Eur. J. Biochem., 163, 653–658. [DOI] [PubMed] [Google Scholar]
- Sun J., Wu,J., Carrasco,N. and Kaback,H.R. (1996) Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry, 35, 990–998. [DOI] [PubMed] [Google Scholar]
- van Geest M. and Lolkema,J.S. (2000) Membrane topology and insertion of membrane proteins: search for topogenic signals. Microbiol. Mol. Biol. Rev., 64, 13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klompenburg W., Nilsson,I., von Heijne,G. and de Kruijff,B. (1997) Anionic phospholipids are determinants of membrane protein topology. EMBO J., 16, 4261–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M.F. and Wilson,T.H. (1996) Molecular biology of the lactose carrier of Escherichia coli. Biochim. Biophys. Acta, 1276, 21–34. [DOI] [PubMed] [Google Scholar]
- Venkatesan P., Hu,Y. and Kaback,H.R. (2000a) Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: helix X. Biochemistry, 39, 10656–10661. [DOI] [PubMed] [Google Scholar]
- Venkatesan P., Kwaw,I., Hu,Y. and Kaback,H.R. (2000b) Site-directed sulfhydryl labeling of lactose permease of Escherichia coli: helix VII. Biochemistry, 39, 10641–10648. [DOI] [PubMed] [Google Scholar]
- Venkatesan P., Liu,Z., Hu,Y. and Kaback,H.R. (2000c) Site-directed sulfhydryl labeling of the lactose permease of Escherichia coli: N-ethylmaleimide-sensitive face of helix II. Biochemistry, 39, 10649–10655. [DOI] [PubMed] [Google Scholar]
- von Heijne G. (1992) Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol., 225, 487–494. [DOI] [PubMed] [Google Scholar]
- Wada T., Long,J.C., Zhang,D. and Vik,S.B. (1999) A novel labeling approach supports the five-transmembrane model of subunit a of the Escherichia coli ATP synthase. J. Biol. Chem., 274, 17353–17357. [DOI] [PubMed] [Google Scholar]
- Weinglass A.B. and Kaback,H.R. (2000) The central cytoplasmic loop of the major facilitator superfamily of transport proteins governs efficient membrane insertion. Proc. Natl Acad. Sci. USA, 97, 8938–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin C.D. and Kaback,H.R. (1999) Estimating loop–helix interfaces in a polytopic membrane protein by deletion analysis. Biochemistry, 38, 8590–8597. [DOI] [PubMed] [Google Scholar]
- Wu J. and Kaback,H.R. (1997) Helix proximity and ligand-induced conformational changes in the lactose permease of Escherichia coli determined by site-directed chemical crosslinking. J. Mol. Biol., 270, 285–293. [DOI] [PubMed] [Google Scholar]
- Wu J., Sun,J. and Kaback,H.R. (1996) Purification and functional characterization of the C-terminal half of the lactose permease of Escherichia coli. Biochemistry, 35, 5213–5219. [DOI] [PubMed] [Google Scholar]
- Yamato I. (1992) Membrane assembly of lactose permease of Escherichia coli. J. Biochem. (Tokyo), 111, 444–450. [DOI] [PubMed] [Google Scholar]
- Zen K.H., Consler,T.G. and Kaback,H.R. (1995) Insertion of the polytopic membrane protein lactose permease occurs by multiple mechanisms. Biochemistry, 34, 3430–3437. [DOI] [PubMed] [Google Scholar]
- Zhu Q.-S., von Dippe,P., Xing,W. and Levy,D. (1999) Membrane topology and cell surface targeting of microsomal epoxide hydrolase. Evidence for multiple topological orientations. J. Biol. Chem., 274, 27898–27904. [DOI] [PubMed] [Google Scholar]