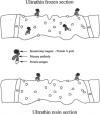
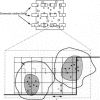
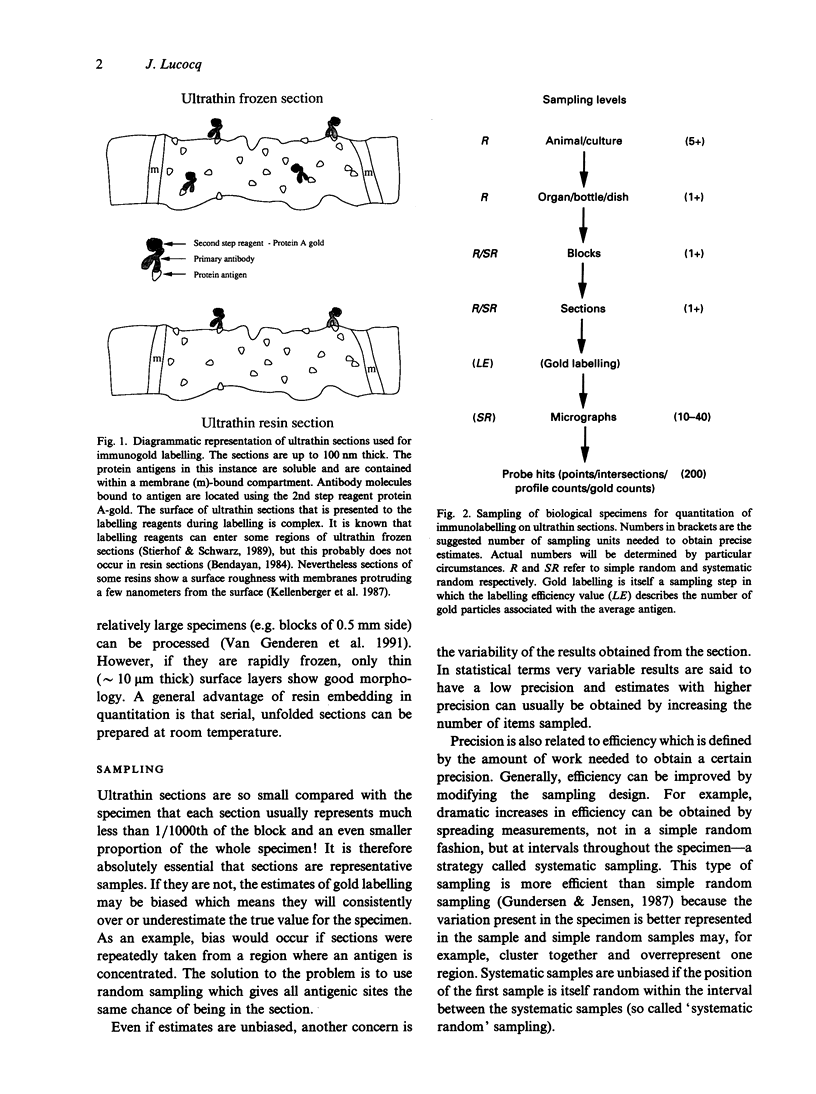
Full text
PDF

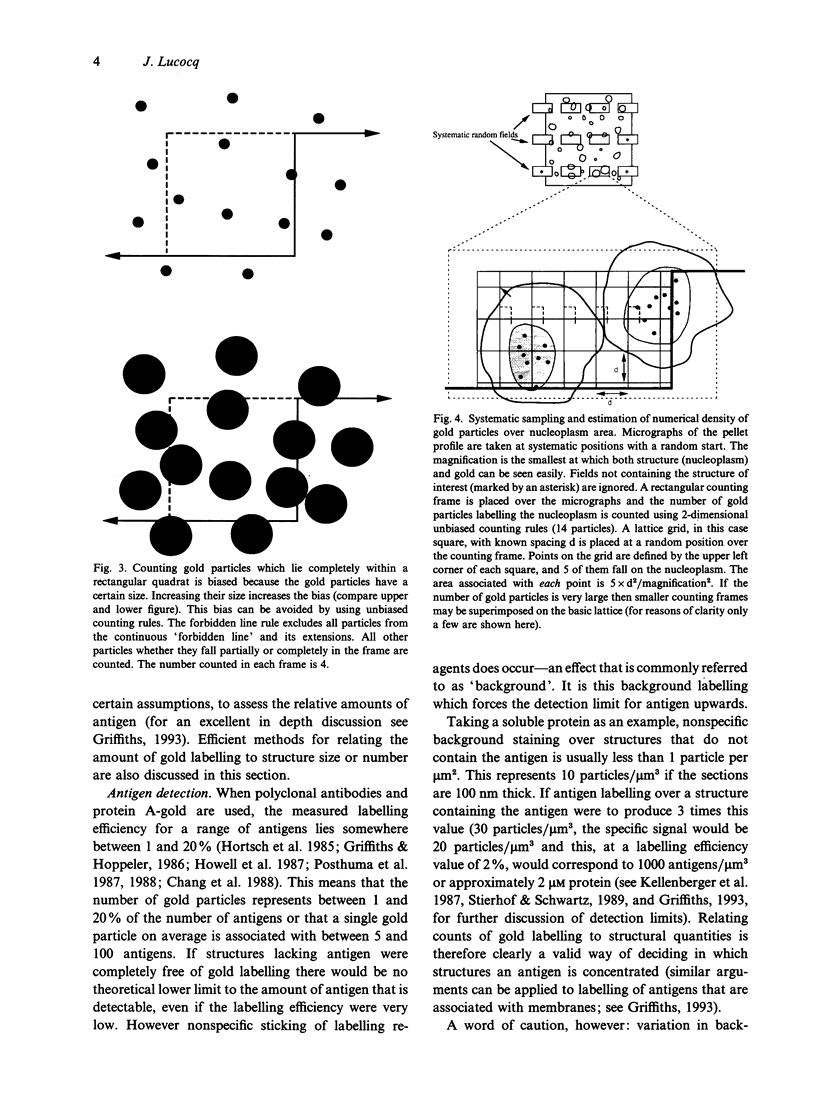
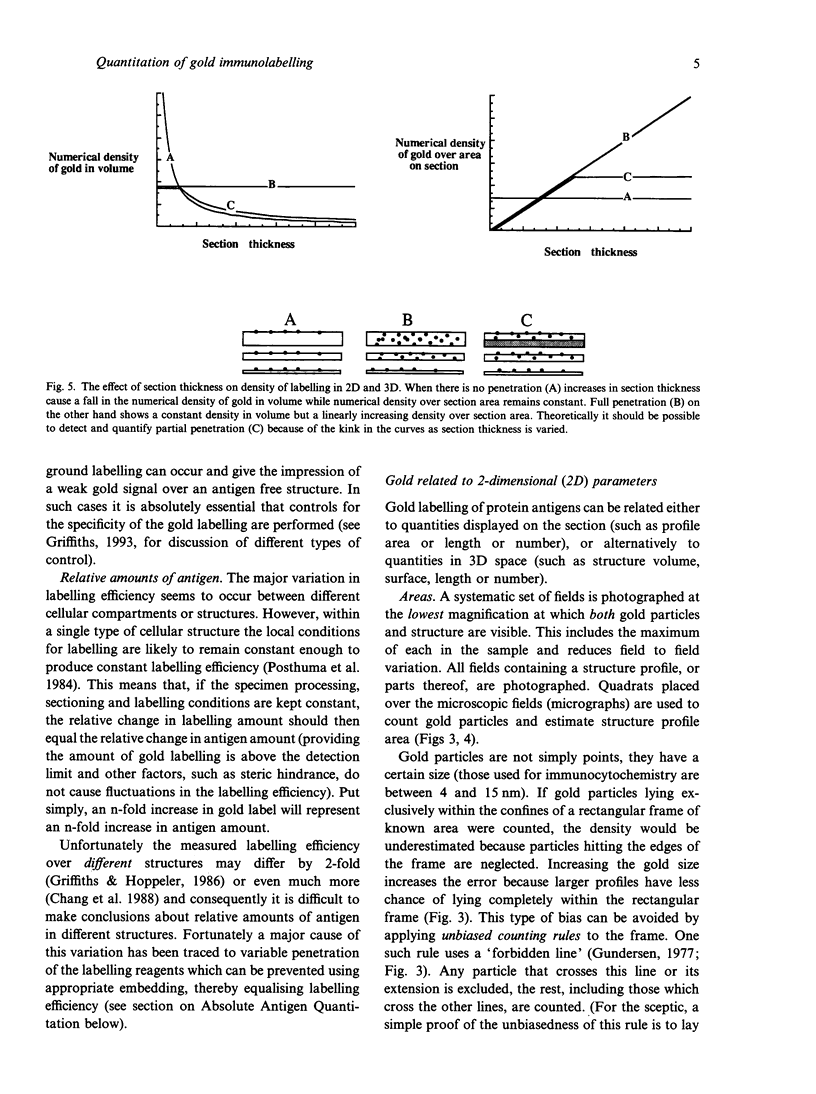





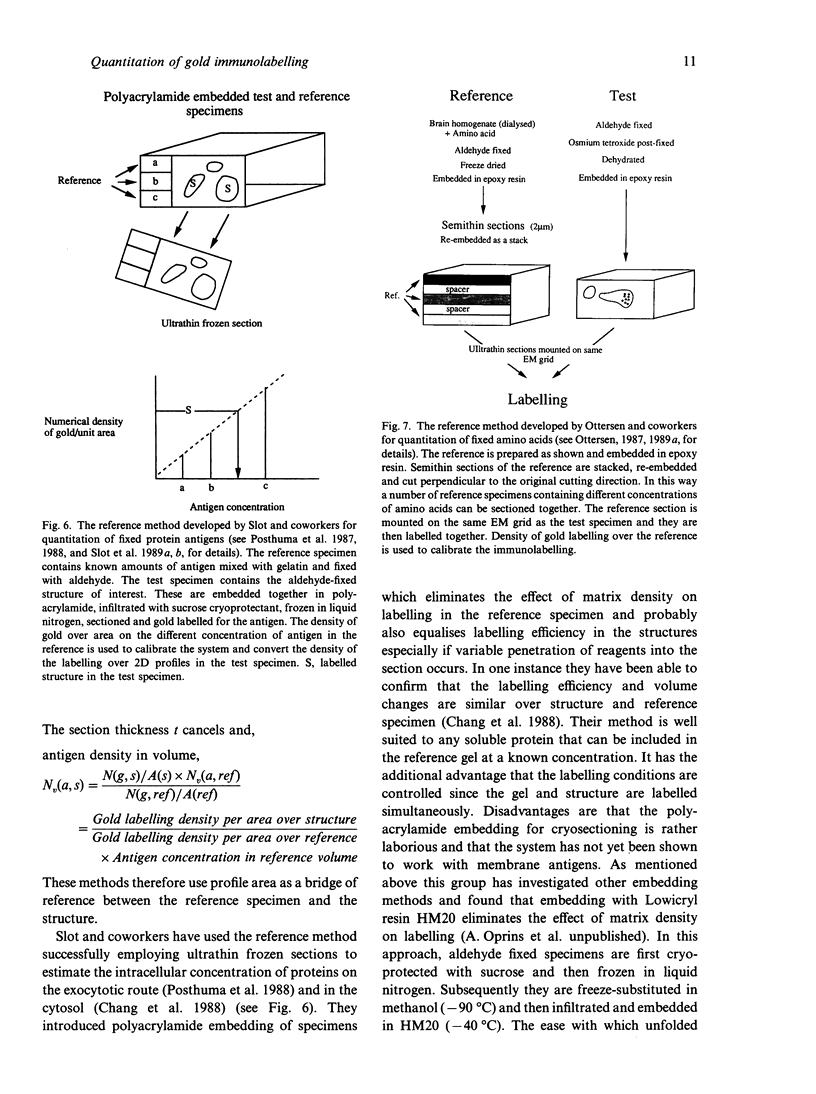


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedi K. S. A simple method of measuring the thickness of semi-thin and ultra-thin sections. J Microsc. 1987 Oct;148(Pt 1):107–111. doi: 10.1111/j.1365-2818.1987.tb02858.x. [DOI] [PubMed] [Google Scholar]
- Braendgaard H., Gundersen H. J. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods. 1986 Oct;18(1-2):39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Broman J., Ottersen O. P. Cervicothalamic tract terminals are enriched in glutamate-like immunoreactivity: an electron microscopic double-labeling study in the cat. J Neurosci. 1992 Jan;12(1):204–221. doi: 10.1523/JNEUROSCI.12-01-00204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B., Walter C., Griffiths G., Warren G. Viral glycoproteins at different stages of intracellular transport can be distinguished using monoclonal antibodies. Eur J Cell Biol. 1983 Sep;31(2):315–324. [PubMed] [Google Scholar]
- Chang L. Y., Slot J. W., Geuze H. J., Crapo J. D. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988 Dec;107(6 Pt 1):2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orive L. M. Particle number can be estimated using a disector of unknown thickness: the selector. J Microsc. 1987 Feb;145(Pt 2):121–142. [PubMed] [Google Scholar]
- Cruz-Orive L. M., Weibel E. R. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990 Apr;258(4 Pt 1):L148–L156. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Hoppeler H. Quantitation in immunocytochemistry: correlation of immunogold labeling to absolute number of membrane antigens. J Histochem Cytochem. 1986 Nov;34(11):1389–1398. doi: 10.1177/34.11.3534077. [DOI] [PubMed] [Google Scholar]
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984 Oct;89(1):65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Quinn P., Mathieu-Costello O., Hoppeler H. Density of newly synthesized plasma membrane proteins in intracellular membranes. I. Stereological studies. J Cell Biol. 1984 Jun;98(6):2133–2141. doi: 10.1083/jcb.98.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H. J., Bagger P., Bendtsen T. F., Evans S. M., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988 Oct;96(10):857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Osterby R. Optimizing sampling efficiency of stereological studies in biology: or 'do more less well!'. J Microsc. 1981 Jan;121(Pt 1):65–73. doi: 10.1111/j.1365-2818.1981.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Gupta M., Mayhew T. M., Bedi K. S., Sharma A. K., White F. H. Inter-animal variation and its influence on the overall precision of morphometric estimates based on nested sampling designs. J Microsc. 1983 Aug;131(Pt 2):147–154. doi: 10.1111/j.1365-2818.1983.tb04241.x. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Griffiths G., Meyer D. I. Restriction of docking protein to the rough endoplasmic reticulum: immunocytochemical localization in rat liver. Eur J Cell Biol. 1985 Sep;38(2):271–279. [PubMed] [Google Scholar]
- Howell K. E., Reuter-Carlson U., Devaney E., Luzio J. P., Fuller S. D. One antigen, one gold? A quantitative analysis of immunogold labeling of plasma membrane 5'-nucleotidase in frozen thin sections. Eur J Cell Biol. 1987 Oct;44(2):318–327. [PubMed] [Google Scholar]
- Ji Z. Q., Aas J. E., Laake J., Walberg F., Ottersen O. P. An electron microscopic, immunogold analysis of glutamate and glutamine in terminals of rat spinocerebellar fibers. J Comp Neurol. 1991 May 8;307(2):296–310. doi: 10.1002/cne.903070210. [DOI] [PubMed] [Google Scholar]
- Jørgen H., Gundersen G., Boysen M., Reith A. Comparison of semiautomatic digitizer-tablet and simple point counting performance in morphometry. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):317–325. doi: 10.1007/BF02892580. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Dürrenberger M., Villiger W., Carlemalm E., Wurtz M. The efficiency of immunolabel on Lowicryl sections compared to theoretical predictions. J Histochem Cytochem. 1987 Sep;35(9):959–969. doi: 10.1177/35.9.3302020. [DOI] [PubMed] [Google Scholar]
- King M. V. Dimensional changes in cells and tissues during specimen preparation for the electron microscope. Cell Biophys. 1991 Feb;18(1):31–55. doi: 10.1007/BF02990514. [DOI] [PubMed] [Google Scholar]
- Lucocq J. Quantitation of gold labeling and estimation of labeling efficiency with a stereological counting method. J Histochem Cytochem. 1992 Dec;40(12):1929–1936. doi: 10.1177/40.12.1453009. [DOI] [PubMed] [Google Scholar]
- Mathieu O., Cruz-Orive L. M., Hoppeler H., Weibel E. R. Measuring error and sampling variation in stereology: comparison of the efficiency of various methods for planar image analysis. J Microsc. 1981 Jan;121(Pt 1):75–88. doi: 10.1111/j.1365-2818.1981.tb01200.x. [DOI] [PubMed] [Google Scholar]
- Mattfeldt T., Mall G., Gharehbaghi H., Möller P. Estimation of surface area and length with the orientator. J Microsc. 1990 Sep;159(Pt 3):301–317. doi: 10.1111/j.1365-2818.1990.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992 May;21(5):313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P. Postembedding immunogold labelling of fixed glutamate: an electron microscopic analysis of the relationship between gold particle density and antigen concentration. J Chem Neuroanat. 1989 Jan-Feb;2(1):57–66. [PubMed] [Google Scholar]
- Ottersen O. P. Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res. 1987;69(1):167–174. doi: 10.1007/BF00247039. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 1989;180(1):1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- Ottersen O. P., Zhang N., Walberg F. Metabolic compartmentation of glutamate and glutamine: morphological evidence obtained by quantitative immunocytochemistry in rat cerebellum. Neuroscience. 1992;46(3):519–534. doi: 10.1016/0306-4522(92)90141-n. [DOI] [PubMed] [Google Scholar]
- Posthuma G., Slot J. W., Geuze H. J. Immunocytochemical assays of amylase and chymotrypsinogen in rat pancreas secretory granules. Efficacy of using immunogold-labeled ultrathin cryosections to estimate relative protein concentrations. J Histochem Cytochem. 1984 Oct;32(10):1028–1034. doi: 10.1177/32.10.6207220. [DOI] [PubMed] [Google Scholar]
- Posthuma G., Slot J. W., Geuze H. J. Usefulness of the immunogold technique in quantitation of a soluble protein in ultra-thin sections. J Histochem Cytochem. 1987 Apr;35(4):405–410. doi: 10.1177/35.4.2434559. [DOI] [PubMed] [Google Scholar]
- Posthuma G., Slot J. W., Veenendaal T., Geuze H. J. Immunogold determination of amylase concentrations in pancreatic subcellular compartments. Eur J Cell Biol. 1988 Jun;46(2):327–335. [PubMed] [Google Scholar]
- Quinn P., Griffiths G., Warren G. Density of newly synthesized plasma membrane proteins in intracellular membranes II. Biochemical studies. J Cell Biol. 1984 Jun;98(6):2142–2147. doi: 10.1083/jcb.98.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Shupliakov O., Brodin L., Cullheim S., Ottersen O. P., Storm-Mathisen J. Immunogold quantification of glutamate in two types of excitatory synapse with different firing patterns. J Neurosci. 1992 Oct;12(10):3789–3803. doi: 10.1523/JNEUROSCI.12-10-03789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Stierhof Y. D., Schwarz H. Labeling properties of sucrose-infiltrated cryosections. Scanning Microsc Suppl. 1989;3:35–46. [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp R., Andiné P., Hagberg H., Karagülle T., Blackstad T. W., Ottersen O. P. Cellular and subcellular redistribution of glutamate-, glutamine- and taurine-like immunoreactivities during forebrain ischemia: a semiquantitative electron microscopic study in rat hippocampus. Neuroscience. 1991;41(2-3):433–447. doi: 10.1016/0306-4522(91)90339-p. [DOI] [PubMed] [Google Scholar]
- Zhang N., Walberg F., Laake J. H., Meldrum B. S., Ottersen O. P. Aspartate-like and glutamate-like immunoreactivities in the inferior olive and climbing fibre system: a light microscopic and semiquantitative electron microscopic study in rat and baboon (Papio anubis). Neuroscience. 1990;38(1):61–80. doi: 10.1016/0306-4522(90)90374-d. [DOI] [PubMed] [Google Scholar]
- van Genderen I. L., van Meer G., Slot J. W., Geuze H. J., Voorhout W. F. Subcellular localization of Forssman glycolipid in epithelial MDCK cells by immuno-electronmicroscopy after freeze-substitution. J Cell Biol. 1991 Nov;115(4):1009–1019. doi: 10.1083/jcb.115.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]