Abstract
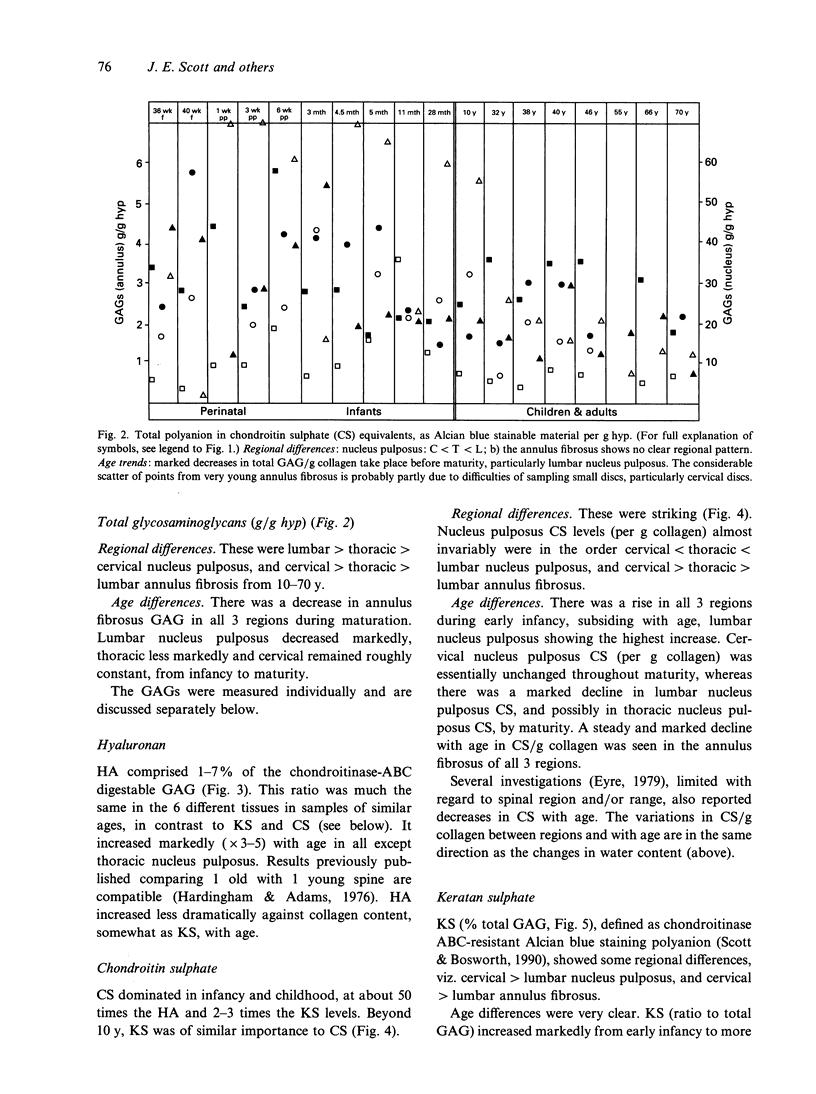
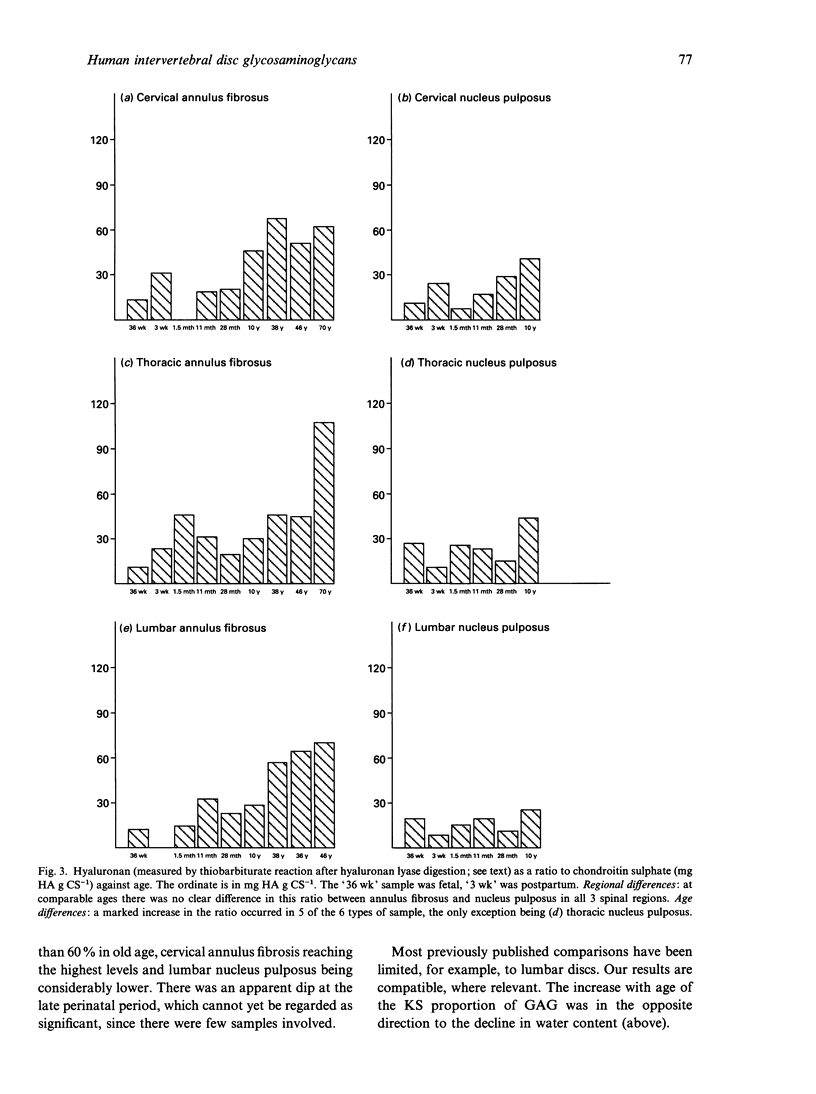
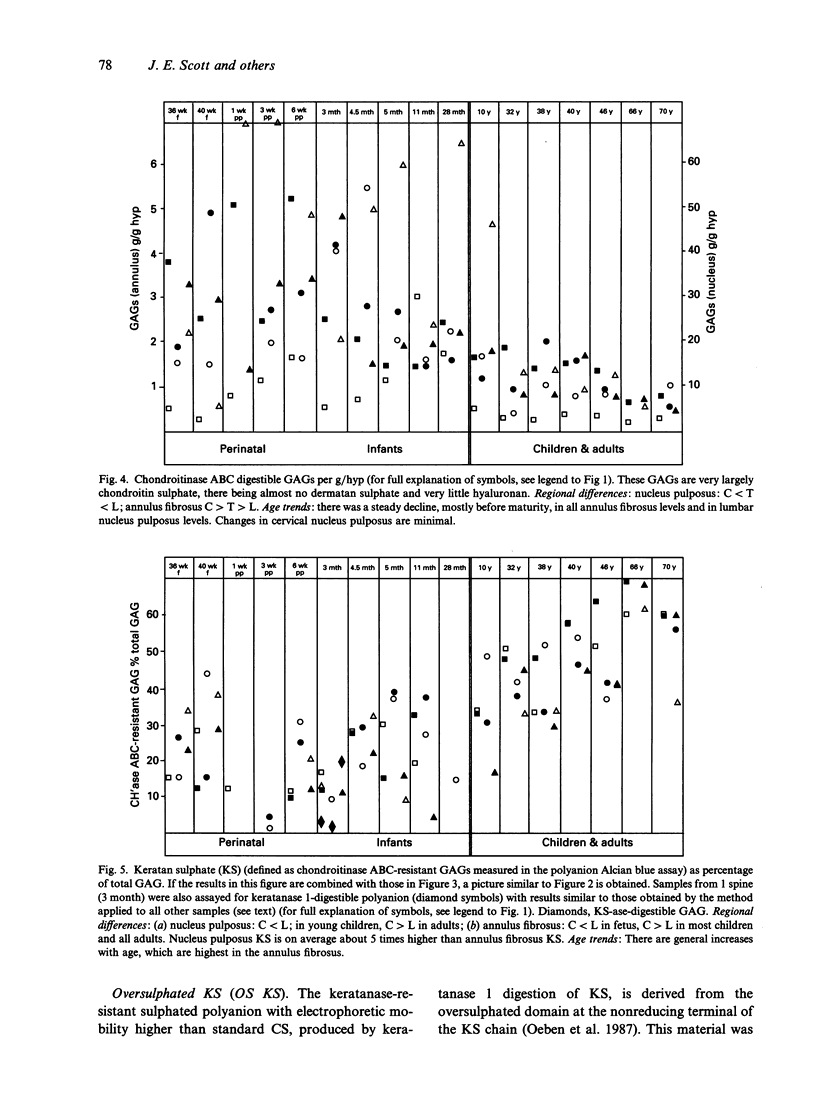
Hyaluronan (HA), chondroitin and keratan sulphates (CS, KS), collagen and dry weights were measured in the annulus fibrosus and nucleus pulposus of human cervical, thoracic and lumbar intervertebral discs aged 36-79 y. Alcian blue-critical electrolyte concentration (CEC) staining of sections extended the results. The collagen, total polyanion, HA, CS and KS contents of the nucleus pulposus and annulus fibrosus were plotted for all 3 regions against age. Regional differences and age-related trends were found. For regional differences, the collagen content of the nucleus pulposus was highest in cervical discs and lowest in lumbar discs. In contrast, the total polyanion content of the nucleus pulposus was highest in lumbar discs and lowest in cervical discs. These differences were seen in fetal and adult discs. With respect to age-related trends, the collagen content of the annulus fibrosus was higher in adults and children than in neonates and infants. The collagen content of the nucleus pulposus increased with age in thoracic and lumbar discs, but it was consistently high in cervical discs. There was generally a downward trend of total polyanion and CS with increase in age. This was quite consistent for the annulus fibrosus in all regions and there were dramatic decreases in the lumbar nucleus pulposus in all adults compared with infants and children. These trends were least evident in the cervical nucleus pulposus where infant values were low. CS changes correlated with water content. HA and KS increased in all discs with increasing maturity. Oversulphated KS, absent from fetal discs, reached mature levels by 10 y. Many of the changes occurred before maturity. Glycosaminoglycan (GAG) levels correlated with increasing compressive loads. Higher collagen levels in the cervical nucleus pulposus correlated with greater ranges of torsional and shearing strains in cervical discs. High GAG levels in cervical annulus fibrosus probably facilitate lamellar movements during torsional and flexional movements by lubrication and increase of tissue compressibility. Increased KS/CS ratios before maturity correlated with decreased disc blood supply. Ambient O2 tensions may determine KS/CS balance, the former consuming little O2 during biosynthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P., Eyre D. R., Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977 Feb;16(1):22–29. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- Balduini C., De Luca G., Passi A., Rindi S., Salvini R., Scott J. E. Effect of oxygen tension and lactate concentration on keratan sulphate and chondroitin sulphate biosynthesis in bovine cornea. Biochim Biophys Acta. 1992 Jan 23;1115(3):187–191. doi: 10.1016/0304-4165(92)90052-v. [DOI] [PubMed] [Google Scholar]
- Emes J. H., Pearce R. H. The proteoglycans of the human intervertebral disc. Biochem J. 1975 Mar;145(3):549–556. doi: 10.1042/bj1450549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- Haigh M., Scott J. E. A method of processing tissue sections for staining with cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30(4):479–486. [PubMed] [Google Scholar]
- Hardingham T. E., Adams P. A method for the determination of hyaluronate in the presence of other glycosaminoglycans and its application to human intervertebral disc. Biochem J. 1976 Oct 1;159(1):143–147. doi: 10.1042/bj1590143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Nachemson A. Recent advances in the treatment of low back pain. Int Orthop. 1985;9(1):1–10. doi: 10.1007/BF00267031. [DOI] [PubMed] [Google Scholar]
- Oeben M., Keller R., Stuhlsatz H. W., Greiling H. Constant and variable domains of different disaccharide structure in corneal keratan sulphate chains. Biochem J. 1987 Nov 15;248(1):85–93. doi: 10.1042/bj2480085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Bosworth T. R. A comparative biochemical and ultrastructural study of proteoglycan-collagen interactions in corneal stroma. Functional and metabolic implications. Biochem J. 1990 Sep 1;270(2):491–497. doi: 10.1042/bj2700491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Chondroitin sulphate and keratan sulphate are almost isosteric. Biochem J. 1991 Apr 1;275(Pt 1):267–268. doi: 10.1042/bj2750267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Keratan sulphate and the ultrastructure of cornea and cartilage: a 'stand-in' for chondroitin sulphate in conditions of oxygen lack? J Anat. 1988 Jun;158:95–108. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan histochemistry--a valuable tool for connective tissue biochemists. Coll Relat Res. 1985 Dec;5(6):541–575. doi: 10.1016/s0174-173x(85)80008-x. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat. 1990 Apr;169:23–35. [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A. Changes in the acid glycosaminoglycan content of the matrix of ageing human articular cartilage. Ann Rheum Dis. 1970 Sep;29(5):509–515. doi: 10.1136/ard.29.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. R., Scott J. E., Cribb A. M., Bosworth T. R. Human intervertebral disc acid glycosaminoglycans. J Anat. 1992 Feb;180(Pt 1):137–141. [PMC free article] [PubMed] [Google Scholar]
- Twomey L., Taylor J. Flexion creep deformation and hysteresis in the lumbar vertebral column. Spine (Phila Pa 1976) 1982 Mar-Apr;7(2):116–122. doi: 10.1097/00007632-198203000-00005. [DOI] [PubMed] [Google Scholar]
- Venn G., Mason R. M. Absence of keratan sulphate from skeletal tissues of mouse and rat. Biochem J. 1985 Jun 1;228(2):443–450. doi: 10.1042/bj2280443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALMSLEY R. The development and growth of the intervertebral disc. Edinb Med J. 1953 Aug;60(8):341–364. [PMC free article] [PubMed] [Google Scholar]
- Webber C., Glant T. T., Roughley P. J., Poole A. R. The identification and characterization of two populations of aggregating proteoglycans of high buoyant density isolated from post-natal human articular cartilages of different ages. Biochem J. 1987 Dec 15;248(3):735–740. doi: 10.1042/bj2480735. [DOI] [PMC free article] [PubMed] [Google Scholar]