Abstract
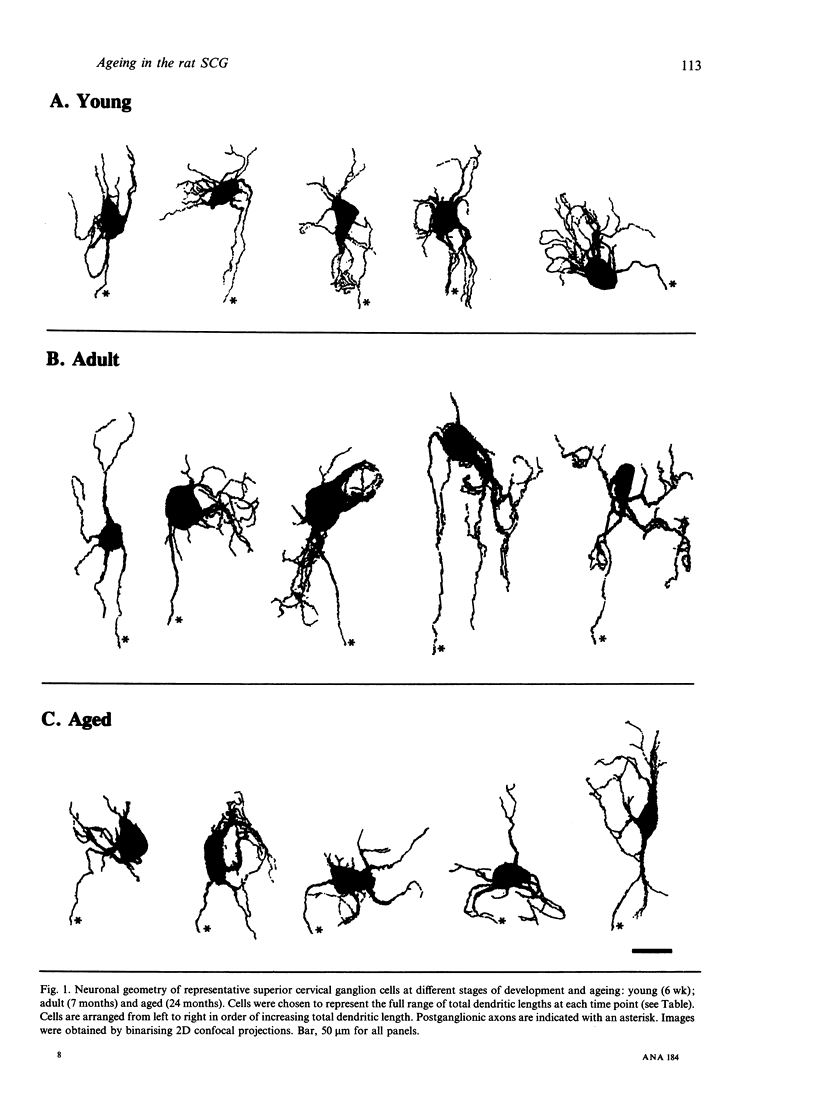
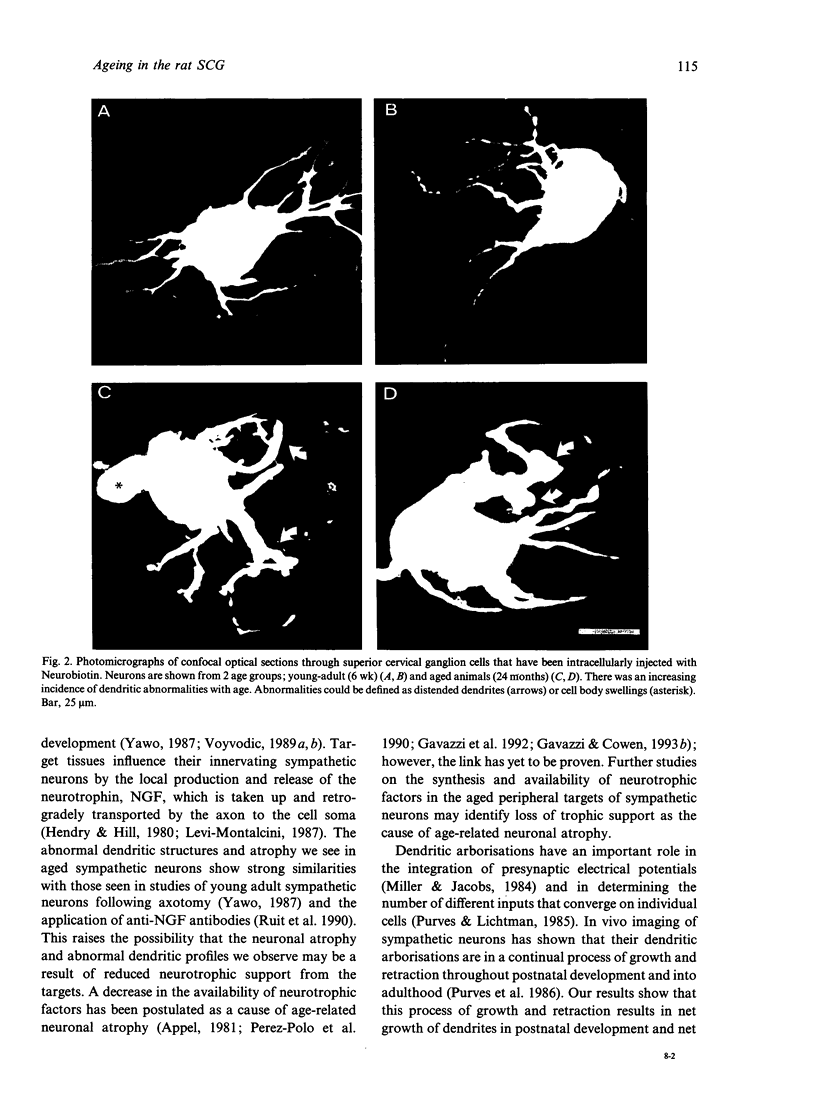
Intracellular injection of a biotinylated probe in fixed superior cervical ganglia followed by confocal microscopy was used to investigate the effects of age on the dendritic arborisation of sympathetic neurons in rats aged 6 wk (young adult), 7 months (fully grown adult) and 24 months (aged). In accordance with other studies considerable dendritic growth was observed during postnatal development. However, in old age dendritic growth did not continue, and significant atrophy was observed. Quantitation of neuronal morphology showed significant reductions in soma size, total dendritic length, number of branch points and total area of dendritic arborisation in old age. Unexpectedly, significant reductions in the numbers of primary dendrites were observed in maturity and in old age. Concomitant with this atrophy there was an increase in age-related morphological abnormalities. The similarities between the atrophy and dendritic abnormalities shown by our aged neurons and those seen in other studies of young adult sympathetic neurons following axotomy or trophic factor deprivation are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Rahman T. A., Collins K. J., Cowen T., Rustin M. Immunohistochemical, morphological and functional changes in the peripheral sudomotor neuro-effector system in elderly people. J Auton Nerv Syst. 1992 Mar;37(3):187–197. doi: 10.1016/0165-1838(92)90040-n. [DOI] [PubMed] [Google Scholar]
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Cowen T., Haven A. J., Wen-Qin C., Gallen D. D., Franc F., Burnstock G. Development and ageing of perivascular adrenergic nerves in the rabbit. A quantitative fluorescence histochemical study using image analysis. J Auton Nerv Syst. 1982 May;5(3):317–336. doi: 10.1016/0165-1838(82)90074-1. [DOI] [PubMed] [Google Scholar]
- Cowen T., Thrasivoulou C. Cerebrovascular nerves in old rats show reduced accumulation of 5-hydroxytryptamine and loss of nerve fibres. Brain Res. 1990 Apr 16;513(2):237–243. doi: 10.1016/0006-8993(90)90461-j. [DOI] [PubMed] [Google Scholar]
- Dhall U., Cowen T., Haven A. J., Burnstock G. Perivascular noradrenergic and peptide-containing nerves show different patterns of changes during development and ageing in the guinea-pig. J Auton Nerv Syst. 1986 Jun;16(2):109–126. doi: 10.1016/0165-1838(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Forehand C. J. Density of somatic innervation on mammalian autonomic ganglion cells is inversely related to dendritic complexity and preganglionic convergence. J Neurosci. 1985 Dec;5(12):3403–3408. doi: 10.1523/JNEUROSCI.05-12-03403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989 Jun;96(6):1487–1493. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Gavazzi I., Andrews T. J., Thrasivoulou C., Cowen T. Influence of target tissues on their innervation in old age: a transplantation study. Neuroreport. 1992 Aug;3(8):717–720. doi: 10.1097/00001756-199208000-00017. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Hill C. E. Retrograde axonal transport of target tissue-derived macromolecules. Nature. 1980 Oct 16;287(5783):647–649. doi: 10.1038/287647a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Nelson V. H. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J Physiol. 1975 Feb;245(1):91–135. doi: 10.1113/jphysiol.1975.sp010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Jacobs G. A. Relationships between neuronal structure and function. J Exp Biol. 1984 Sep;112:129–145. doi: 10.1242/jeb.112.1.129. [DOI] [PubMed] [Google Scholar]
- Mione M. C., Dhital K. K., Amenta F., Burnstock G. An increase in the expression of neuropeptidergic vasodilator, but not vasoconstrictor, cerebrovascular nerves in aging rats. Brain Res. 1988 Sep 13;460(1):103–113. doi: 10.1016/0006-8993(88)91210-3. [DOI] [PubMed] [Google Scholar]
- Njå A., Purves D. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J Physiol. 1978 Apr;277:53–75. [PMC free article] [PubMed] [Google Scholar]
- Purves D., Hadley R. D., Voyvodic J. T. Dynamic changes in the dendritic geometry of individual neurons visualized over periods of up to three months in the superior cervical ganglion of living mice. J Neurosci. 1986 Apr;6(4):1051–1060. doi: 10.1523/JNEUROSCI.06-04-01051.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Geometrical differences among homologous neurons in mammals. Science. 1985 Apr 19;228(4697):298–302. doi: 10.1126/science.3983631. [DOI] [PubMed] [Google Scholar]
- Purves D., Snider W. D., Voyvodic J. T. Trophic regulation of nerve cell morphology and innervation in the autonomic nervous system. Nature. 1988 Nov 10;336(6195):123–128. doi: 10.1038/336123a0. [DOI] [PubMed] [Google Scholar]
- Ruit K. G., Osborne P. A., Schmidt R. E., Johnson E. M., Jr, Snider W. D. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. J Neurosci. 1990 Jul;10(7):2412–2419. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruit K. G., Snider W. D. Administration or deprivation of nerve growth factor during development permanently alters neuronal geometry. J Comp Neurol. 1991 Dec 1;314(1):106–113. doi: 10.1002/cne.903140110. [DOI] [PubMed] [Google Scholar]
- Santer R. M., Baker D. M. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988 Nov;25(1):59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Santer R. M. Morphological evidence for the maintenance of the cervical sympathetic system in aged rats. Neurosci Lett. 1991 Sep 16;130(2):248–250. doi: 10.1016/0304-3940(91)90407-k. [DOI] [PubMed] [Google Scholar]
- Snider W. D. Rostrocaudal differences in dendritic growth and synaptogenesis in rat sympathetic chain ganglia. J Comp Neurol. 1986 Feb 8;244(2):245–253. doi: 10.1002/cne.902440210. [DOI] [PubMed] [Google Scholar]
- Voyvodic J. T. Development and regulation of dendrites in the rat superior cervical ganglion. J Neurosci. 1987 Mar;7(3):904–912. doi: 10.1523/JNEUROSCI.07-03-00904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic J. T. Peripheral target regulation of dendritic geometry in the rat superior cervical ganglion. J Neurosci. 1989 Jun;9(6):1997–2010. doi: 10.1523/JNEUROSCI.09-06-01997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic J. T. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989 Nov 23;342(6248):430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- Yawo H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J Neurosci. 1987 Nov;7(11):3703–3711. doi: 10.1523/JNEUROSCI.07-11-03703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]