Abstract
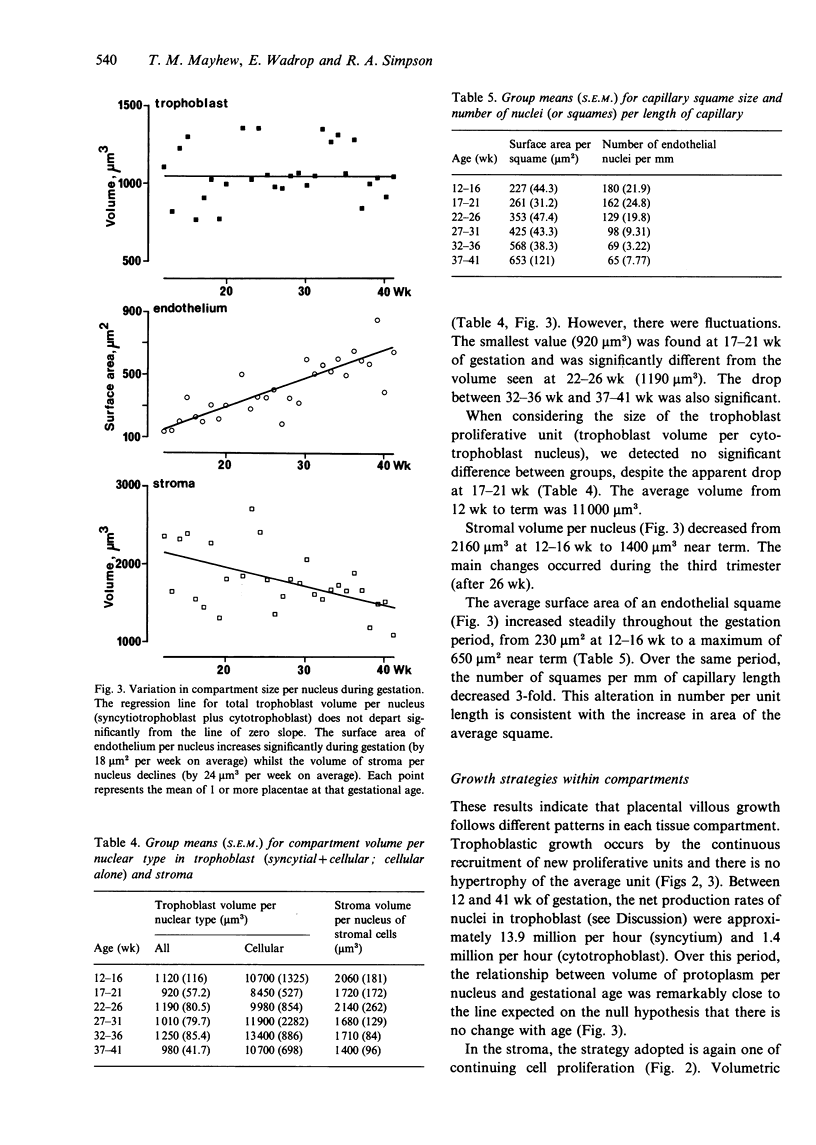
A cross-sectional sample of human placentae was collected at 12-41 wk, and growth mechanisms within villous subcompartments (trophoblast epithelium, stroma and fetal capillary endothelium) were assessed using state-of-the-art design-based stereological methods. Physical disectors (adjacent pairs of sections) were used to count nuclei in syncytiotrophoblast and in cells of the cytotrophoblast, stroma and endothelium. The volumes of trophoblast and stroma and the surface areas and lengths of capillaries were employed to assess the overall growth of each compartment. Growth within compartments was monitored as total number of nuclei (a measure of nuclear proliferation) and compartment size per nucleus (a measure of hypertrophy). During gestation, all compartments grew dramatically. Numbers of nuclei increased exponentially and followed an uninhibited growth model. Volumetric growth of the placenta did not keep pace with the increase in total nuclear number (all types). Growth of trophoblast was exclusively hyperplastic and proceeded at an average net recruitment rate of 15 million nuclei per hour with a numerical predominance of syncytiotrophoblast nuclei of 9:1. There was no evidence of hypertrophy: trophoblast volume per nucleus averaged 1080 microns 3. Growth within stroma involved cell proliferation (17 million nuclei per hour) and the volume of stroma per nucleus declined at a rate of about 24 microns 3 per week. Capillary growth was hyperplastic (5 million endothelial squames per hour) and, possibly, hypertrophic (the mean area of a squame increased by about 18 microns 2 per week). Linear growth of vessels exceeded cellular recruitment and the density of squames along the capillaries decreased each week by about 5 per mm.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonds D. R., Mwape B., Kumar S., Gabbe S. G. Human fetal weight and placental weight growth curves. A mathematical analysis from a population at sea level. Biol Neonate. 1984;45(6):261–274. doi: 10.1159/000242016. [DOI] [PubMed] [Google Scholar]
- Boyd P. A., Brown R. A., Coghill G. R., Slidders W., Stewart W. J. Measurement of the mass of syncytiotrophoblast in a range of human placentae using an image analysing computer. Placenta. 1983 Jul-Sep;4(3):255–262. doi: 10.1016/s0143-4004(83)80004-6. [DOI] [PubMed] [Google Scholar]
- Boyd P. A. Quantitative structure of the normal human placenta from 10 weeks of gestation to term. Early Hum Dev. 1984 Jun;9(4):297–307. doi: 10.1016/0378-3782(84)90074-4. [DOI] [PubMed] [Google Scholar]
- Castellucci M., Kaufmann P. Evolution of the stroma in human chorionic villi throughout pregnancy. Bibl Anat. 1982;(22):40–45. [PubMed] [Google Scholar]
- Castellucci M., Zaccheo D., Pescetto G. A three-dimensional study of the normal human placental villous core. I. The Hofbauer cells. Cell Tissue Res. 1980;210(2):235–247. doi: 10.1007/BF00237612. [DOI] [PubMed] [Google Scholar]
- Enders A. C., King B. F. The cytology of Hofbauer cells. Anat Rec. 1970 Jun;167(2):231–236. doi: 10.1002/ar.1091670211. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Jensen E. B. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987 Sep;147(Pt 3):229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986 Jul;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Iyengar L. Chemical composition of placenta in pregnancies with small-for-date infants. Am J Obstet Gynecol. 1973 May 1;116(1):66–70. doi: 10.1016/0002-9378(73)90885-5. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Mayhew T. M., Boyd P. A. Quantitative description of the elaboration and maturation of villi from 10 weeks of gestation to term. Placenta. 1992 Jul-Aug;13(4):357–370. doi: 10.1016/0143-4004(92)90060-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Bruns U., Leiser R., Luckhardt M., Winterhager E. The fetal vascularisation of term human placental villi. II. Intermediate and terminal villi. Anat Embryol (Berl) 1985;173(2):203–214. doi: 10.1007/BF00316301. [DOI] [PubMed] [Google Scholar]
- Kaufmann P. Development and differentiation of the human placental villous tree. Bibl Anat. 1982;(22):29–39. [PubMed] [Google Scholar]
- Kaufmann P., Stark J., Stegner H. E. The villous stroma of the human placenta. I. The ultrastructure of fixed connective tissue cells. Cell Tissue Res. 1977 Feb 2;177(1):105–121. doi: 10.1007/BF00221122. [DOI] [PubMed] [Google Scholar]
- Laga E. M., Driscoll S. G., Munro H. N. Quantitative studies of human placenta. II. Biochemical characteristics. Biol Neonate. 1973;23(3):260–283. doi: 10.1159/000240606. [DOI] [PubMed] [Google Scholar]
- Laird A. K. Dynamics of embryonic growth. Growth. 1966 Jun;30(2):263–275. [PubMed] [Google Scholar]
- Mayhew T. M. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992 May;21(5):313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Mayhew T. M., Burton G. J. Methodological problems in placental morphometry: apologia for the use of stereology based on sound sampling practice. Placenta. 1988 Nov-Dec;9(6):565–581. doi: 10.1016/0143-4004(88)90001-x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Morris R. J. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- Potten C. S. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 1974 Jan;7(1):77–88. doi: 10.1111/j.1365-2184.1974.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Sands J., Dobbing J. Continuing growth and development of the third-trimester human placenta. Placenta. 1985 Jan-Feb;6(1):13–21. doi: 10.1016/s0143-4004(85)80028-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. A., Mayhew T. M., Barnes P. R. From 13 weeks to term, the trophoblast of human placenta grows by the continuous recruitment of new proliferative units: a study of nuclear number using the disector. Placenta. 1992 Sep-Oct;13(5):501–512. doi: 10.1016/0143-4004(92)90055-x. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Stringer B. M., Wynford-Thomas D., Williams E. D. Physical randomization of tissue architecture: an alternative to systemic sampling. J Microsc. 1982 May;126(Pt 2):179–182. doi: 10.1111/j.1365-2818.1982.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Functional significance of the zonal morphologic differences in the normal human placenta. A morphometric study. Am J Obstet Gynecol. 1978 Apr 1;130(7):773–781. doi: 10.1016/0002-9378(78)90007-8. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Gestational changes in the functional structure of the human placenta in relation to fetal growth: a morphometric study. Am J Obstet Gynecol. 1980 Jul 1;137(5):560–568. doi: 10.1016/0002-9378(80)90696-1. [DOI] [PubMed] [Google Scholar]
- Ward B. S. Cellular growth of the placenta in twin pregnancy late in gestation. Placenta. 1985 Mar-Apr;6(2):107–116. doi: 10.1016/s0143-4004(85)80061-8. [DOI] [PubMed] [Google Scholar]
- Winick M., Coscia A., Noble A. Cellular growth in human placenta. I. Normal placental growth. Pediatrics. 1967 Feb;39(2):248–251. [PubMed] [Google Scholar]
- Zajicek G. The intestinal proliferon. J Theor Biol. 1977 Aug 7;67(3):515–521. doi: 10.1016/0022-5193(77)90053-4. [DOI] [PubMed] [Google Scholar]
- Ziegler E. E., O'Donnell A. M., Nelson S. E., Fomon S. J. Body composition of the reference fetus. Growth. 1976 Dec;40(4):329–341. [PubMed] [Google Scholar]



