Abstract
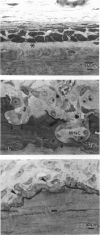
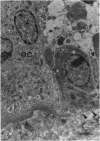
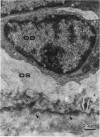
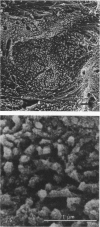
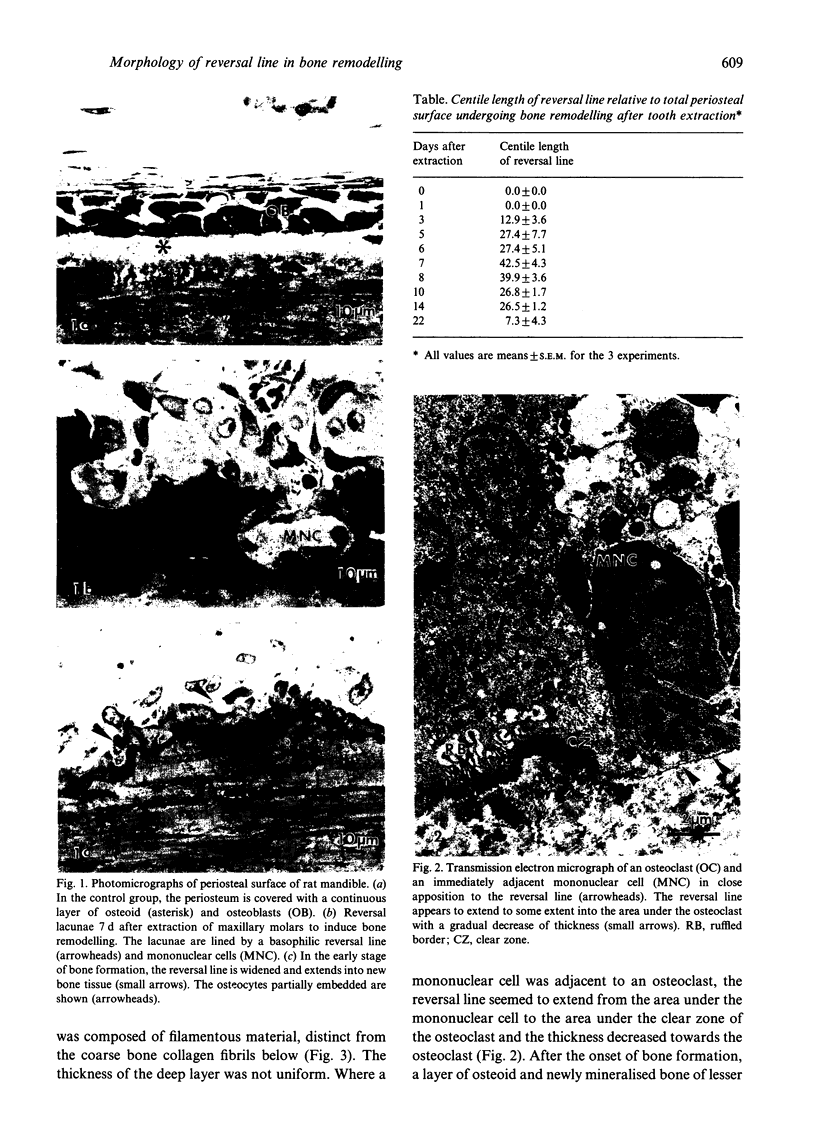
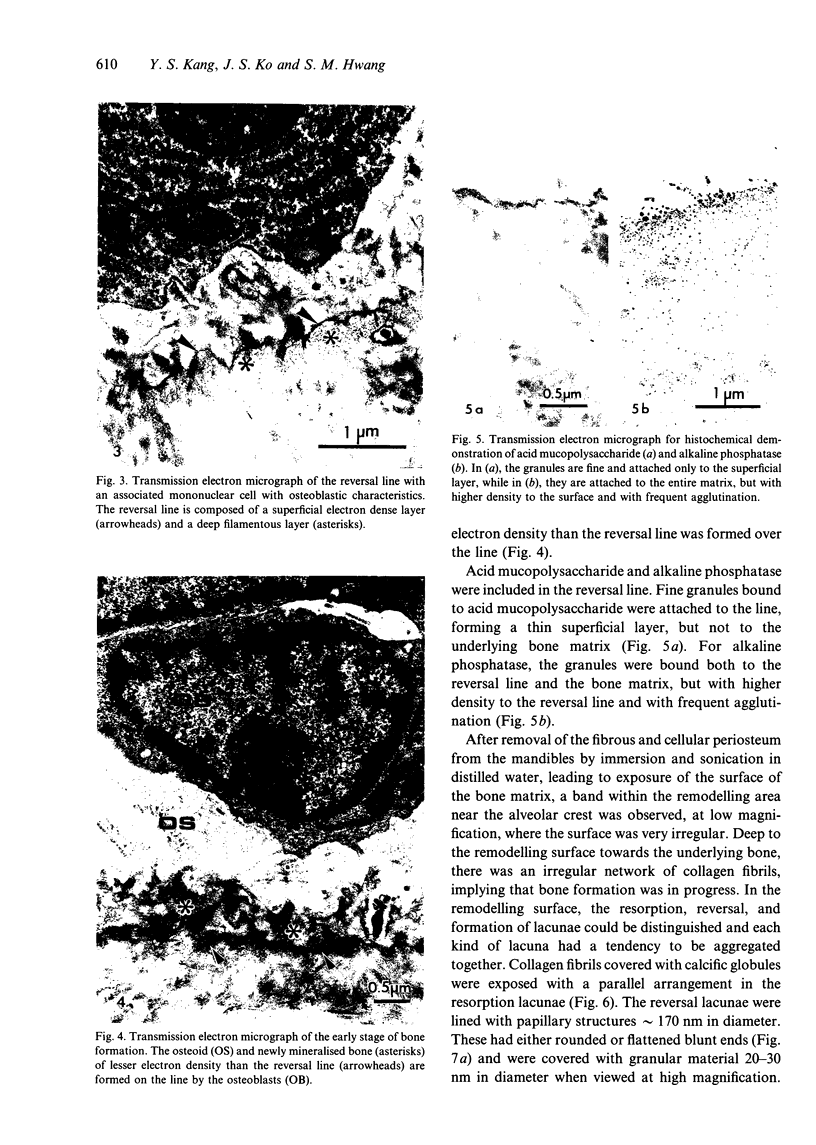
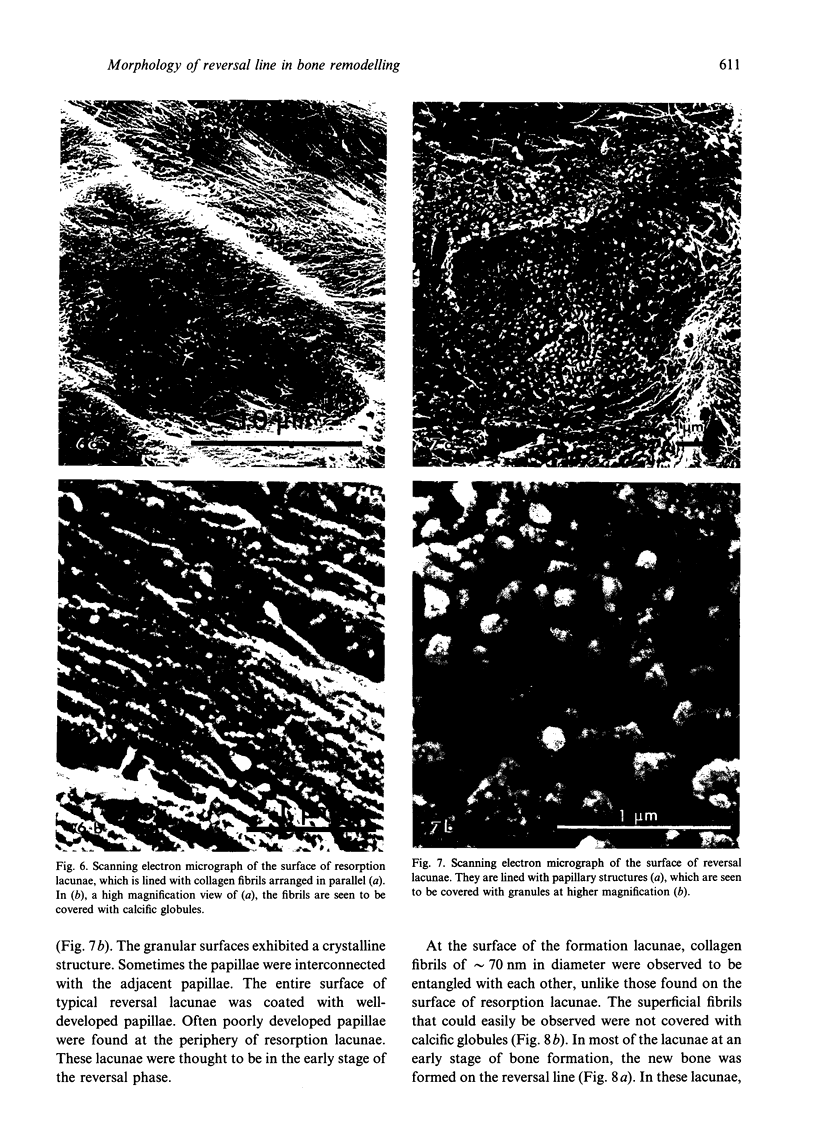
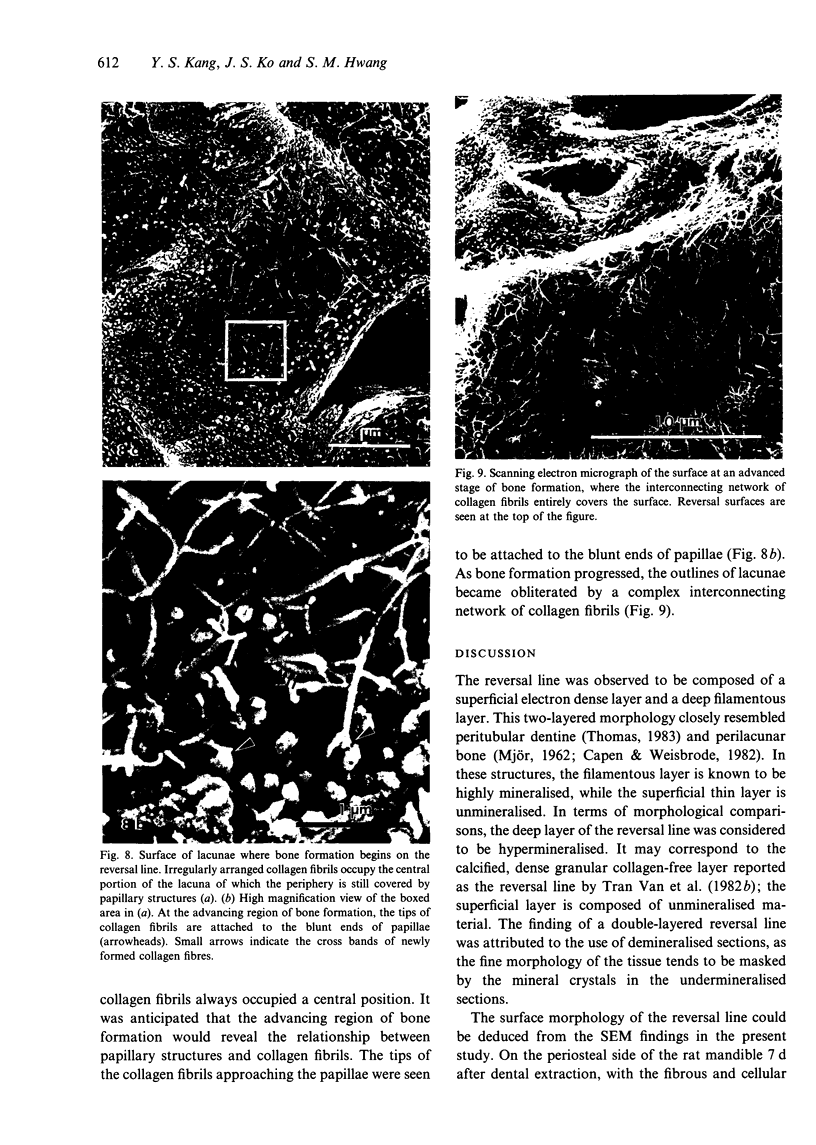
The ultrastructure of the reversal line during alveolar bone remodelling was investigated in the rat. Surface bone remodelling along the periosteum of the mandible was induced by the extraction of opposing maxillary molars. With transmission EM the reversal line was seen to be composed of a superficial electron-dense amorphous layer and a deep filamentous layer at 7 d after extraction. The reversal line exhibited strong alkaline phosphatase activity and contained acid mucopolysaccharide. Scanning EM of the surface of the line, exposed by sonication in distilled water, showed papillary structures, the surface of which appeared granular and exhibited a crystalline appearance. The tips of collagen fibrils of new bone were attached to the top of the papillae in the front area of bone formation. It is suggested that the reversal line is involved in the coupling of bone resorption and formation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers T. J. The pathobiology of the osteoclast. J Clin Pathol. 1985 Mar;38(3):241–252. doi: 10.1136/jcp.38.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., McCulloch C. A., Sodek J. Bone sialoprotein in developing porcine dental tissues: cellular expression and comparison of tissue localization with osteopontin and osteonectin. Arch Oral Biol. 1993 Mar;38(3):241–249. doi: 10.1016/0003-9969(93)90034-j. [DOI] [PubMed] [Google Scholar]
- Huffer W. E. Morphology and biochemistry of bone remodeling: possible control by vitamin D, parathyroid hormone, and other substances. Lab Invest. 1988 Oct;59(4):418–442. [PubMed] [Google Scholar]
- Kurahashi Y., Yoshiki S. Electron microscopic localization of alkaline phosphatase in the enamel organ of the young rat. Arch Oral Biol. 1972 Jan;17(1):155–163. doi: 10.1016/0003-9969(72)90143-4. [DOI] [PubMed] [Google Scholar]
- Owen M., Shetlar M. R. Uptake of 3H-glucosamine by osteoclasts. Nature. 1968 Dec 28;220(5174):1335–1336. doi: 10.1038/2201335a0. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism. 1976 Jul;25(7):809–844. doi: 10.1016/0026-0495(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int. 1984;36 (Suppl 1):S37–S45. doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- RINEHART J. F., ABUL-HAJ S. K. An improved method for histologic demonstration of acid mucopolysaccharides in tissues. AMA Arch Pathol. 1951 Aug;52(2):189–194. [PubMed] [Google Scholar]
- Sauren Y. M., Mieremet R. H., Groot C. G., Scherft J. P. An electron microscopic study on the presence of proteoglycans in the mineralized matrix of rat and human compact lamellar bone. Anat Rec. 1992 Jan;232(1):36–44. doi: 10.1002/ar.1092320105. [DOI] [PubMed] [Google Scholar]
- Schaffler M. B., Burr D. B., Frederickson R. G. Morphology of the osteonal cement line in human bone. Anat Rec. 1987 Mar;217(3):223–228. doi: 10.1002/ar.1092170302. [DOI] [PubMed] [Google Scholar]
- Scherft J. P. The lamina limitans of the organic matrix of calcified cartilage and bone. J Ultrastruct Res. 1972 Feb;38(3):318–331. doi: 10.1016/s0022-5320(72)90008-1. [DOI] [PubMed] [Google Scholar]
- Thomas H. F. The effect of various fixatives on the extent of the odontoblast process in human dentine. Arch Oral Biol. 1983;28(5):465–469. doi: 10.1016/0003-9969(83)90145-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. R., Baylink D. J., Wergedal J. E. Increases in number and size of osteoclasts in response to calcium or phosphorus deficiency in the rat. Endocrinology. 1975 Aug;97(2):283–289. doi: 10.1210/endo-97-2-283. [DOI] [PubMed] [Google Scholar]
- Tran Van P. T., Vignery A., Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982 Apr;202(4):445–451. doi: 10.1002/ar.1092020403. [DOI] [PubMed] [Google Scholar]
- Tran Van P., Vignery A., Baron R. An electron-microscopic study of the bone-remodeling sequence in the rat. Cell Tissue Res. 1982;225(2):283–292. doi: 10.1007/BF00214682. [DOI] [PubMed] [Google Scholar]