Abstract
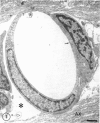
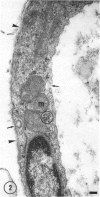
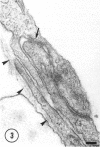
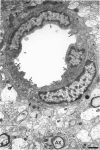
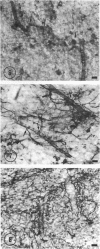
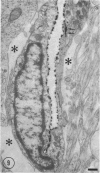
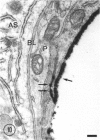
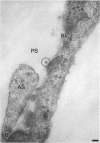
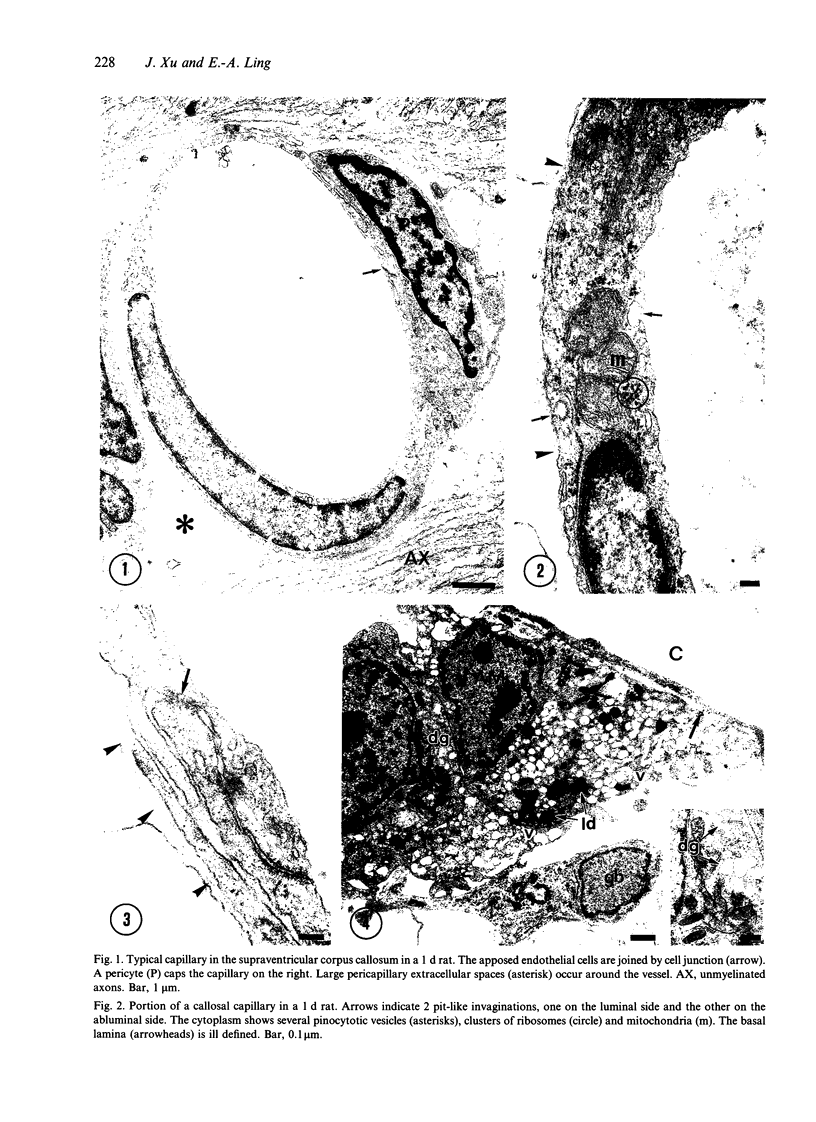
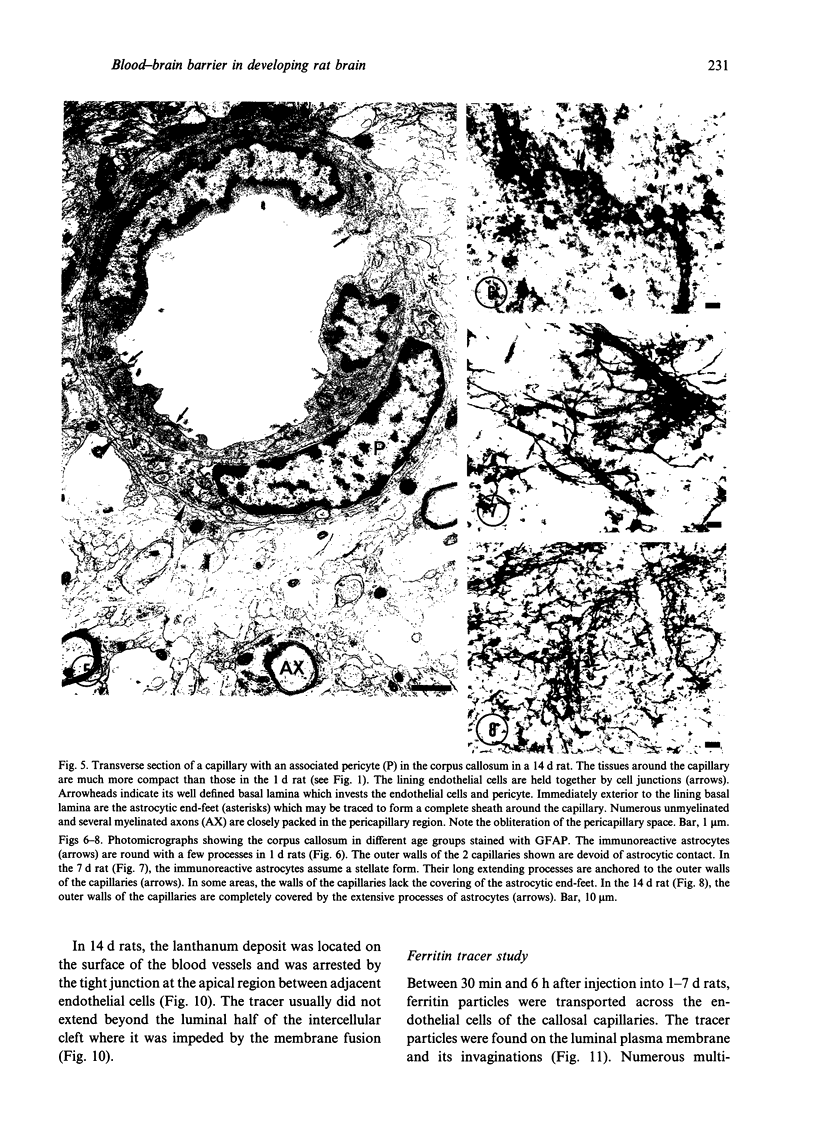
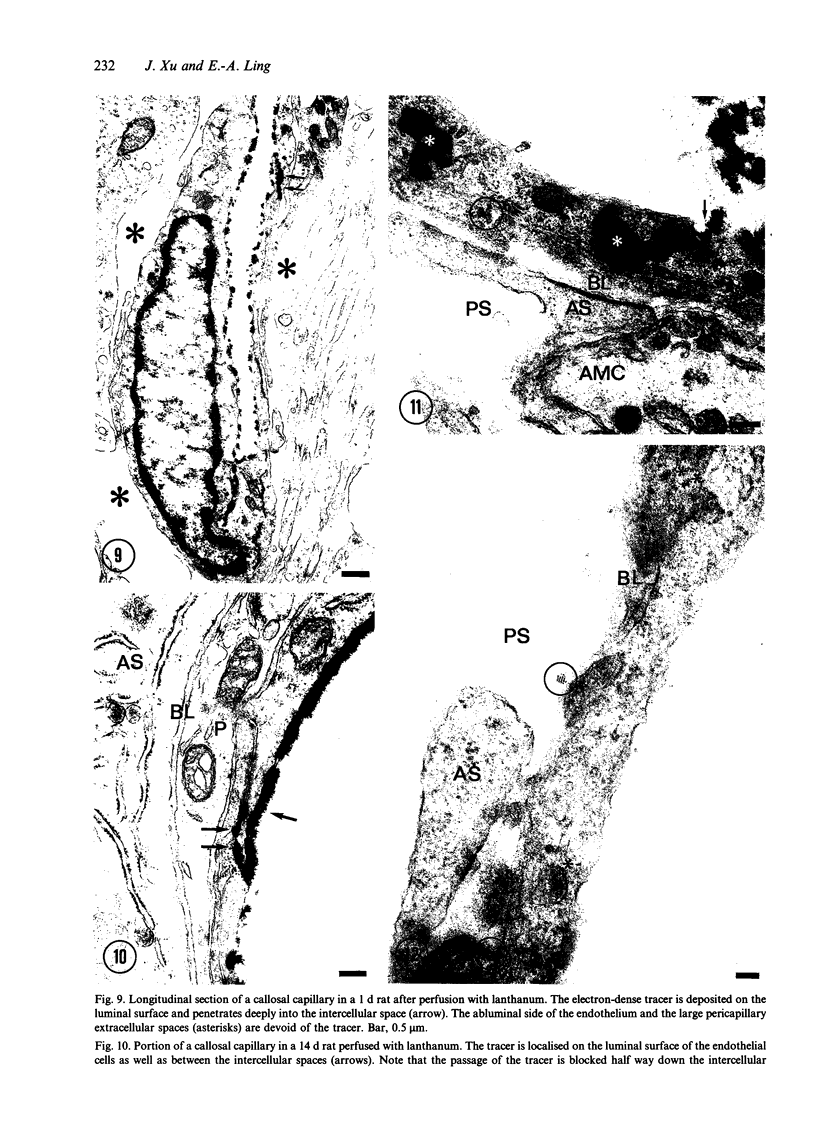
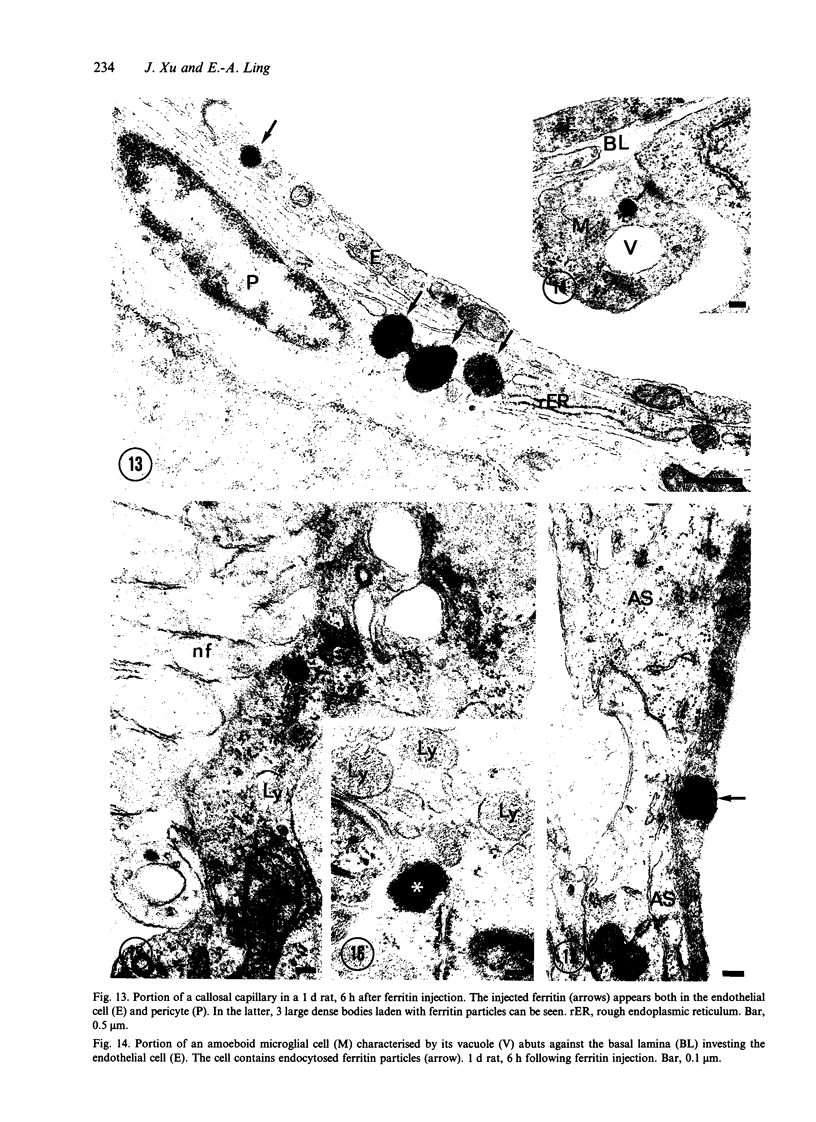
The ultrastructure of the capillaries and their permeability to lanthanum ions and ferritin in the corpus callosum was examined in postnatal rat brain. In 1 and 7-d-old rats, numerous pinocytotic vesicles were observed in the endothelial cytoplasm of the callosal capillaries. Tight junctions were present between adjacent endothelial cells which were surrounded by an ill defined layer of basal lamina. The latter was almost devoid of astrocytic association in 1 d rats and partially covered by astrocytic end-feet in 7 d rats. Pericytes were a common feature. Amoeboid microglial cells were in direct contact with some parts of the vascular wall. Large extracellular spaces were present around the capillaries. In 14 d rats, the walls of the callosal capillaries became more well developed and were surrounded by a continuous sheath of astrocytic end-feet. The basal lamina became denser and well defined. The pericapillary spaces had diminished. Immunostaining for GFAP confirmed that, with age, the walls of the callosal capillaries became increasingly covered by the astrocytic end-feet. After perfusion with lanthanum, the tracer was deposited on the luminal surface but not in the abluminal side of the endothelial cells; the passage of the tracer was apparently obstructed in the intercellular space by the tight junctions in both the 1 and 14 d rats. When injected intravenously in 1-7 d rats, ferritin was transported across the endothelial cells by transcytosis and consequently taken up by the pericytes and amoeboid microglial cells contacting the basal lamina. In 14 d rats, the injected ferritin was only found in the endothelial cytoplasm. It was concluded that the difference in the capillary permeability to exogenous material in 1-7 and 14 d rats is due to the difference in the activities of the transendothelial transport. The pericytes and amoeboid microglial cells associated with the capillary probably play a role as phagocytes in maintaining the function of the blood-brain barrier by trapping any serum-derived foreign substances with astrocytes having a regulatory role in the formation of the barrier.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz A. L., Goldstein G. W. Developmental changes in metabolism and transport properties of capillaries isolated from rat brain. J Physiol. 1981 Mar;312:365–376. doi: 10.1113/jphysiol.1981.sp013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin T. W., Krigman M. R. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res. 1975 Dec 5;99(2):444–448. doi: 10.1016/0006-8993(75)90053-0. [DOI] [PubMed] [Google Scholar]
- Brightman M. W. Morphology of blood-brain interfaces. Exp Eye Res. 1977;25 (Suppl):1–25. doi: 10.1016/s0014-4835(77)80008-0. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M. Ultrastructure of frog cerebral and pial microvessels and their impermeability to lanthanum ions. Brain Res. 1982 Jun 3;241(1):57–65. doi: 10.1016/0006-8993(82)91228-8. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J Comp Neurol. 1970 Jan;138(1):31–47. doi: 10.1002/cne.901380104. [DOI] [PubMed] [Google Scholar]
- Cancilla P. A., Baker R. N., Pollock P. S., Frommes S. P. The reaction of pericytes of the central nervous system to exogenous protein. Lab Invest. 1972 Apr;26(4):376–383. [PubMed] [Google Scholar]
- Chau Y. P., Chien C. L., Lu K. S. The permeability of capillaries among the small granule-containing cells in rat superior cervical ganglia: an ultrastructural lanthanum tracer study. Histol Histopathol. 1991 Apr;6(2):261–268. [PubMed] [Google Scholar]
- Chien C. L., Chau Y. P., Lu K. S. Ultrastructural studies on the barrier properties of the paraganglia in the rat recurrent laryngeal nerve. Acta Anat (Basel) 1991;141(3):262–268. doi: 10.1159/000147132. [DOI] [PubMed] [Google Scholar]
- DONAHUE S., PAPPAS G. D. The fine structure of capillaries in the cerebral cortex of the rat at various stages of development. Am J Anat. 1961 May;108:331–347. doi: 10.1002/aja.1001080307. [DOI] [PubMed] [Google Scholar]
- DePace D. M. Distribution of intravascularly injected lanthanum ions in ganglia of the autonomic nervous system of the rat. J Auton Nerv Syst. 1984 Dec;11(4):339–347. doi: 10.1016/0165-1838(84)90082-1. [DOI] [PubMed] [Google Scholar]
- Delorme P., Gayet J., Grignon G. Ultrastructural study on transcapillary exchanges in the developing telencephalon of the chicken. Brain Res. 1970 Sep 16;22(3):269–283. doi: 10.1016/0006-8993(70)90471-3. [DOI] [PubMed] [Google Scholar]
- Janzer R. C., Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987 Jan 15;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Cytochemical localisation of 5'-nucleotidase in amoeboid microglial cells in postnatal rats. J Anat. 1984 Aug;139(Pt 1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Accumulation of protein tracers in pericytes of the central nervous system following systemic injection in immature mice. Acta Neurol Scand. 1973;49(2):189–194. doi: 10.1111/j.1600-0404.1973.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Leong S. K., Shieh J. Y., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in the supraventricular corpus callosum following the application of horseradish peroxidase in the cerebrum and spinal cord in rats. J Anat. 1983 Mar;136(Pt 2):367–377. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Wong W. C. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993 Jan;7(1):9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Lossinsky A. S., Vorbrodt A. W., Wisniewski H. M. Characterization of endothelial cell transport in the developing mouse blood-brain barrier. Dev Neurosci. 1986;8(2):61–75. doi: 10.1159/000112242. [DOI] [PubMed] [Google Scholar]
- MacKenzie M. L., Ghabriel M. N., Allt G. The blood-nerve barrier: an in vivo lanthanum tracer study. J Anat. 1987 Oct;154:27–37. [PMC free article] [PubMed] [Google Scholar]
- Machen T. E., Erlij D., Wooding F. B. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972 Aug;54(2):302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollgøard K., Saunders N. R. Complex tight junctions of epithelial and of endothelial cells in early foetal brain. J Neurocytol. 1975 Aug;4(4):453–468. doi: 10.1007/BF01261375. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. The blood-brain barrier. Exp Eye Res. 1977;25 (Suppl):177–190. doi: 10.1016/s0014-4835(77)80016-x. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990 May;13(5):174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Saunders N. R. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25 (Suppl):523–550. doi: 10.1016/s0014-4835(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Seta K., Sershen H., Lajtha A. Cerebral amino acid uptake in vivo in newborn mice. Brain Res. 1972 Dec 12;47(2):415–425. doi: 10.1016/0006-8993(72)90649-x. [DOI] [PubMed] [Google Scholar]
- Shaklai M., Tavassoli M. Lanthanum as an electron microscopic stain. J Histochem Cytochem. 1982 Dec;30(12):1325–1330. doi: 10.1177/30.12.6185564. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Wiley M. J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981 May;84(1):183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W., Lossinsky A. S., Wisniewski H. M. Localization of alkaline phosphatase activity in endothelia of developing and mature mouse blood-brain barrier. Dev Neurosci. 1986;8(1):1–13. doi: 10.1159/000112236. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Wen C. Y., Shieh J. Y., Ling E. A. A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat. 1992 Dec;181(Pt 3):423–430. [PMC free article] [PubMed] [Google Scholar]
- Xu J., Kaur C., Ling E. A. Variation with age in the labelling of amoeboid microglial cells in rats following intraperitoneal or intravenous injection of a fluorescent dye. J Anat. 1993 Feb;182(Pt 1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Zanetta J. P., Dontenwill M., Bricca G. Postnatal development of the endothelial system of the rat cerebellum using immunohistochemical techniques. Brain Res. 1985 Jan;349(1-2):253–257. doi: 10.1016/0165-3806(85)90149-x. [DOI] [PubMed] [Google Scholar]
- van Deurs B. Observations on the blood-brain barrier in hypertensive rats, with particular reference to phagocytic pericytes. J Ultrastruct Res. 1976 Jul;56(1):65–77. doi: 10.1016/s0022-5320(76)80141-4. [DOI] [PubMed] [Google Scholar]
- van Deurs B. Structural aspects of brain barriers, with special reference to the permeability of the cerebral endothelium and choroidal epithelium. Int Rev Cytol. 1980;65:117–191. doi: 10.1016/s0074-7696(08)61960-9. [DOI] [PubMed] [Google Scholar]