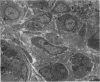
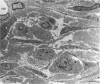
Abstract
It is known that regenerating axons emerging from the proximal stump of a transected nerve are attracted towards the distal stump. It is not certain whether this neurotropic effect is on the axons themselves or whether it is on supporting cells such as Schwann cells that the axons then follow. In order to investigate this question in rats, segments of the sciatic nerve were either isolated or removed and reinserted as grafts, and then sutured into the opposing ends of double-Y silicone tubes. In these tubes, a central conduit was formed by connecting the centrally facing limb of each Y tube. The nerve segments were sutured into one of the limbs at either end. The third limbs of the Y tubes formed side arms, one of which was left open; a plug of mobilised fatty connective tissue was sutured into the other. A gap of 6 mm was left between the cut ends and the fat pads (or openings from the side arms). After 2-3 wk a significantly greater outgrowth (P < 0.001) was found to link the nerve segments than to invade the side arms. The major cell component in the outgrowth was Schwann cells, supported by fibroblasts and capillaries and surrounded by a lamellated layer of flattened fibroblasts. The growth into the side arms had a looser cellular architecture and contained considerably fewer Schwann cells. The results strongly suggest the existence of mutual attraction between emigrant Schwann cells, or possibly endoneurial fibroblasts, from the 2 cut ends of transected nerves. This conclusion has implications for the guidance of axons across gaps in nerves. It does not exclude an additional neurotropic effect from the distal stump on axons.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy D. A., Rud A., Thomas P. K. Neurotropic influence of the distal stump of transected peripheral nerve on axonal regeneration: absence of topographic specificity in adult nerve. J Anat. 1992 Jun;180(Pt 3):395–400. [PMC free article] [PubMed] [Google Scholar]
- Anderson P. N., Turmaine M. Axonal regeneration through arterial grafts. J Anat. 1986 Aug;147:73–82. [PMC free article] [PubMed] [Google Scholar]
- Boyne A. F. A gentle bounce-free assembly for quick-freezing tissues for electron microscopy: application to isolated torpedine ray electrocyte stacks. J Neurosci Methods. 1979 Dec;1(4):353–364. doi: 10.1016/0165-0270(79)90024-4. [DOI] [PubMed] [Google Scholar]
- Bray G. M., Peyronnard J. M., Aguayo A. J. Reactions of unmyelinated nerve fibers to injury. An ultrastructural study. Brain Res. 1972 Jul 20;42(2):297–309. doi: 10.1016/0006-8993(72)90532-x. [DOI] [PubMed] [Google Scholar]
- Bunge M. B., Wood P. M., Tynan L. B., Bates M. L., Sanes J. R. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science. 1989 Jan 13;243(4888):229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- Jurecka W., Ammerer H. P., Lassmann H. Regeneration of a transected peripheral nerve. An autoradiographic and electron microscopic study. Acta Neuropathol. 1975 Oct 1;32(4):299–312. doi: 10.1007/BF00696792. [DOI] [PubMed] [Google Scholar]
- LEE J. C. Electron microscopy of Wallerian degeneration. J Comp Neurol. 1963 Feb;120:65–79. doi: 10.1002/cne.901200107. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Coggeshall R. E. The use of potassium ferricyanide in neural fixation. Anat Rec. 1980 Jul;197(3):297–303. doi: 10.1002/ar.1091970304. [DOI] [PubMed] [Google Scholar]
- Lundborg G., Dahlin L. B., Danielsen N., Nachemson A. K. Tissue specificity in nerve regeneration. Scand J Plast Reconstr Surg. 1986;20(3):279–283. doi: 10.3109/02844318609004486. [DOI] [PubMed] [Google Scholar]
- Ochoa J. Recognition of unmyelinated fiber disease: morphologic criteria. Muscle Nerve. 1978 Sep-Oct;1(5):375–387. doi: 10.1002/mus.880010506. [DOI] [PubMed] [Google Scholar]
- Payer A. F. An ultrastructural study of Schwann cell response to axonal degeneration. J Comp Neurol. 1979 Jan 15;183(2):365–383. doi: 10.1002/cne.901830209. [DOI] [PubMed] [Google Scholar]
- Politis M. J., Ederle K., Spencer P. S. Tropism in nerve regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Res. 1982 Dec 16;253(1-2):1–12. doi: 10.1016/0006-8993(82)90667-9. [DOI] [PubMed] [Google Scholar]
- Scherer S. S., Easter S. S., Jr Degenerative and regenerative changes in the trochlear nerve of goldfish. J Neurocytol. 1984 Aug;13(4):519–565. doi: 10.1007/BF01148079. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Weinberg H. J., Krygier-Brévart V., Zabrenetzky V. Anin vivo method to prepare normal Schwann cells free of axons and myelin. Brain Res. 1979 Apr 6;165(1):119–126. doi: 10.1016/0006-8993(79)90049-0. [DOI] [PubMed] [Google Scholar]
- Steinbrecht R. A., Zierold K. A cryoembedding method for cutting ultrathin cryosections from small frozen specimens. J Microsc. 1984 Oct;136(Pt 1):69–75. doi: 10.1111/j.1365-2818.1984.tb02546.x. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. THE DEPOSITION OF COLLAGEN IN RELATION TO SCHWANN CELL BASEMENT MEMBRANE DURING PERIPHERAL NERVE REGENERATION. J Cell Biol. 1964 Nov;23:375–382. doi: 10.1083/jcb.23.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W., Bisby M. A., Kreutzberg G. W. Changes in cytoskeletal proteins in the rat facial nucleus following axotomy. J Neurosci. 1988 Sep;8(9):3181–3189. doi: 10.1523/JNEUROSCI.08-09-03181.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. K., Jones D. G. The cellular response to nerve injury. II. Regeneration of the perineurium after nerve section. J Anat. 1967 Jan;101(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Thomas P. K. The cellular response to nerve injury. 1. The cellular outgrowth from the distal stump of transected nerve. J Anat. 1966 Apr;100(Pt 2):287–303. [PMC free article] [PubMed] [Google Scholar]
- Tohyama K., Ide C., Nitatori T., Yokota R. Nearest-neighbor distance of intermediate filaments in axons and Schwann cells. Distinction between axons and schwann cell processes in the denervated and reinnervated peripheral nerves. Acta Neuropathol. 1983;60(3-4):194–198. doi: 10.1007/BF00691866. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J. ELECTRON MICROSCOPY AFTER RAPID FREEZING ON A METAL SURFACE AND SUBSTITUTION FIXATION. Anat Rec. 1964 Jul;149:381–385. doi: 10.1002/ar.1091490307. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978 Oct;7(5):555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Longo F. M., Powell H. C., Lundborg G., Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983 Aug 20;218(4):460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]