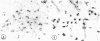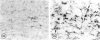Abstract
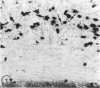
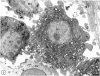
The effects of bacterial lipopolysaccharide (LPS) on the expression of surface antigens including major histocompatibility complex (MHC) and complement type 3 (CR3) receptors on microglial cells in the corpus callosum in postnatal rat brain were investigated. When LPS was injected intravenously (i.v.) in 1-d-old rats, the immunostaining of callosal amoeboid microglial cells with OX-18 directed against MHC class I antigen was enhanced 24 h after the injection in comparison with the controls. The expression of MHC class II (Ia) antigen on the same cell type as shown by its immunoreactivity with OX-6 was also elicited especially after 2 intraperitoneal (i.p.) injections of LPS. Thus 7 d after a single i.p. injection of LPS into 1-d-old rats, only a few OX-6 positive cells showing a moderate staining reaction were observed in the corpus callosum. The immunoreactivity diminished 14 d after the injection. However, in rats receiving 2 successive i.p. injections of LPS at 1 and 4 d of age and killed 7 d after the 1st injection, a significant number of intensely stained OX-6 positive amoeboid microglial cells were observed in the corpus callosum. The expression of MHC class II antigens induced by 2 injections of LPS was sustained at least until d 14 when the callosal ramified microglial cells, known to be derived from gradual metamorphic transformation of amoeboid microglia, still exhibited intense immunoreactivity with OX-6. The effect of LPS on the expression of CR3 on amoeboid microglial cells was not obvious after a single injection, but the immunoreactivity with OX-42 was also augmented in rats given 2 i.p. administration of LPS into rats at 1 an 4 d of age. It is concluded from this study that the expression of MHC class I and class II antigens on amoeboid microglial cells in corpus callosum was upregulated and induced respectively after i.v. or i.p. injection of LPS into early postnatal rats. Although relatively fewer in number when compared with OX-18 and OX-42 positive cells, it is suggested that the OX-6 positive cells would have the potentiality to function in antigen presentation in the postnatal rat brain when challenged by the endotoxin.
Full text
PDF
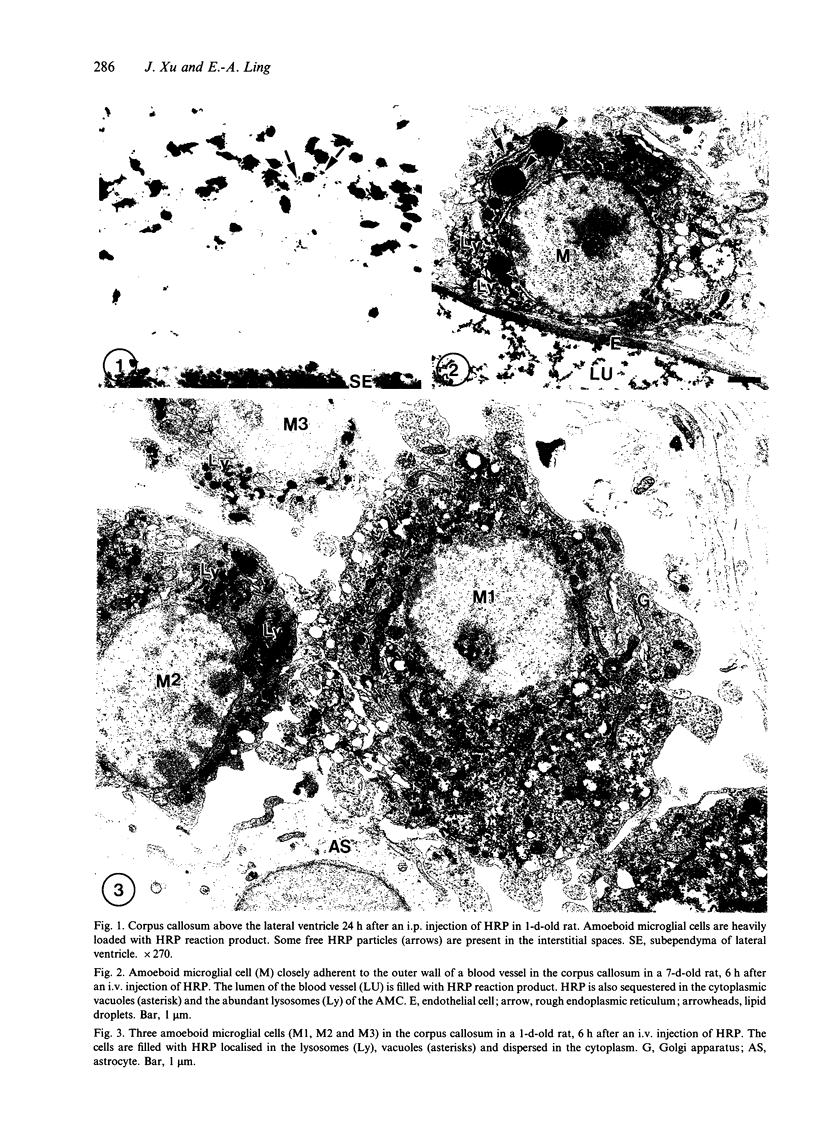


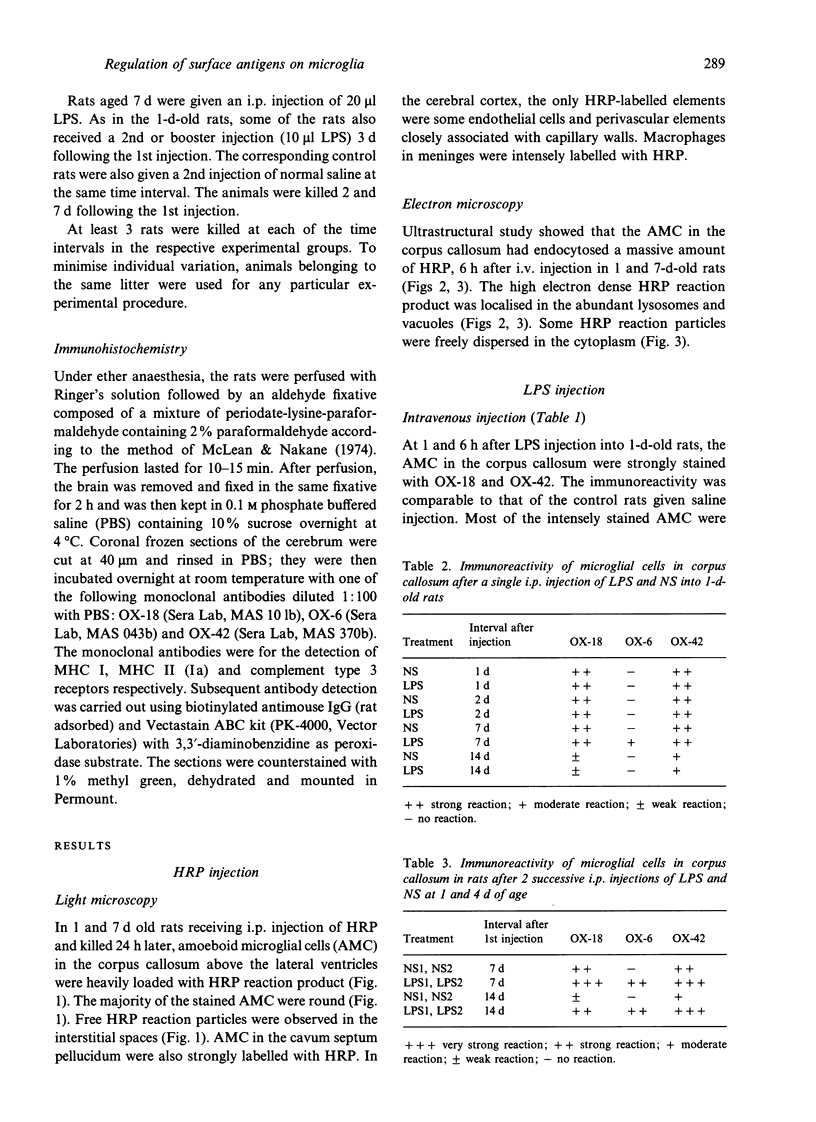



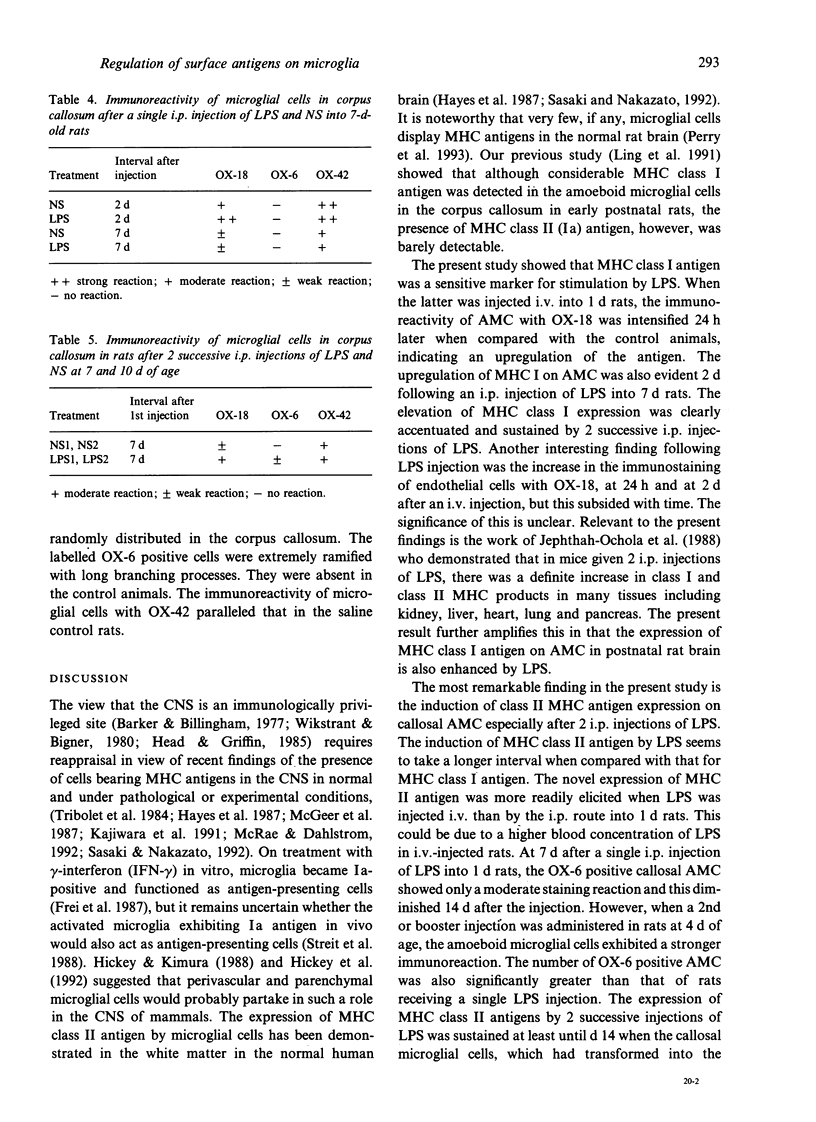



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker C. F., Billingham R. E. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986 Feb 1;136(3):963–970. [PubMed] [Google Scholar]
- De Tribolet N., Hamou M. F., Mach J. P., Carrel S., Schreyer M. Demonstration of HLA-DR antigens in normal human brain. J Neurol Neurosurg Psychiatry. 1984 Apr;47(4):417–418. doi: 10.1136/jnnp.47.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Lee S. C., Mattiace L. A., Yen S. H., Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993 Jan;7(1):75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Yang S. T., Morrison D. C., Betz S. J., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978 Aug 1;148(2):557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsen B. R., Jørgensen M. B., Diemer N. H., Zimmer J. Microglial MHC antigen expression after ischemic and kainic acid lesions of the adult rat hippocampus. Glia. 1993 Jan;7(1):41–49. doi: 10.1002/glia.440070109. [DOI] [PubMed] [Google Scholar]
- Finsen B. R., Sørensen T., Castellano B., Pedersen E. B., Zimmer J. Leukocyte infiltration and glial reactions in xenografts of mouse brain tissue undergoing rejection in the adult rat brain. A light and electron microscopical immunocytochemical study. J Neuroimmunol. 1991 May;32(2):159–183. doi: 10.1016/0165-5728(91)90008-u. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Gehrmann J., Gold R., Linington C., Lannes-Vieira J., Wekerle H., Kreutzberg G. W. Microglial involvement in experimental autoimmune inflammation of the central and peripheral nervous system. Glia. 1993 Jan;7(1):50–59. doi: 10.1002/glia.440070110. [DOI] [PubMed] [Google Scholar]
- Halloran P. F., Urmson J., Farkas S., Phillips R. A., Fulop G., Cockfield S., Autenried P. Effects of cyclosporine on systemic MHC expression. Evidence that non-T cells produce interferon-gamma in vivo and are inhibitable by cyclosporine. Transplantation. 1988 Aug;46(2 Suppl):68S–72S. [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Head J. R., Griffin W. S. Functional capacity of solid tissue transplants in the brain: evidence for immunological privilege. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):375–387. doi: 10.1098/rspb.1985.0039. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Vass K., Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992 May;51(3):246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Ijzermans J. N., Marquet R. L. Interferon-gamma: a review. Immunobiology. 1989 Oct;179(4-5):456–473. doi: 10.1016/S0171-2985(89)80049-X. [DOI] [PubMed] [Google Scholar]
- Jephthah-Ochola J., Urmson J., Farkas S., Halloran P. F. Regulation of MHC expression in vivo. Bacterial lipopolysaccharide induces class I and II MHC products in mouse tissues by a T cell-independent, cyclosporine-sensitive mechanism. J Immunol. 1988 Aug 1;141(3):792–800. [PubMed] [Google Scholar]
- Kajiwara K., Hirozane A., Fukumoto T., Orita T., Nishizaki T., Kamiryo T., Ito H. Major histocompatibility complex expression in brain of rats with graft-versus-host disease. J Neuroimmunol. 1991 Jun;32(3):191–198. doi: 10.1016/0165-5728(91)90188-d. [DOI] [PubMed] [Google Scholar]
- Kaur C., Ling E. A. Activation and re-expression of surface antigen in microglia following an epidural application of kainic acid in the rat brain. J Anat. 1992 Apr;180(Pt 2):333–342. [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Ling E. A., Wong W. C. Labelling of amoeboid microglial cells in rats of various ages following an intravenous injection of horseradish peroxidase. Acta Anat (Basel) 1986;125(2):132–137. doi: 10.1159/000146150. [DOI] [PubMed] [Google Scholar]
- Konno H., Yamamoto T., Iwasaki Y., Suzuki H., Saito T., Terunuma H. Wallerian degeneration induces Ia-antigen expression in the rat brain. J Neuroimmunol. 1989 Dec;25(2-3):151–159. doi: 10.1016/0165-5728(89)90132-x. [DOI] [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Expression of major histocompatibility complex and leukocyte common antigens in amoeboid microglia in postnatal rats. J Anat. 1991 Aug;177:117–126. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Expression of major histocompatibility complex antigens and CR3 complement receptors in activated microglia following an injection of ricin into the sciatic nerve in rats. Histol Histopathol. 1992 Jan;7(1):93–100. [PubMed] [Google Scholar]
- Ling E. A., Kaur L. C., Yick T. Y., Wong W. C. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anat Embryol (Berl) 1990;182(5):481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Wong W. C. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993 Jan;7(1):9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Tago H., McGeer E. G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987 Aug 18;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Kawamata T., Walker D. G., Akiyama H., Tooyama I., McGeer E. G. Microglia in degenerative neurological disease. Glia. 1993 Jan;7(1):84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Butcher G. W., Hämmerling G. J. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-gamma. J Immunol. 1986 Feb 1;136(3):940–948. [PubMed] [Google Scholar]
- Morioka T., Streit W. J. Expression of immunomolecules on microglial cells following neonatal sciatic nerve axotomy. J Neuroimmunol. 1991 Dec;35(1-3):21–30. doi: 10.1016/0165-5728(91)90158-4. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Matyszak M. K., Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993 Jan;7(1):60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Poltorak M., Freed W. J. Immunological reactions induced by intracerebral transplantation: evidence that host microglia but not astroglia are the antigen-presenting cells. Exp Neurol. 1989 Mar;103(3):222–233. doi: 10.1016/0014-4886(89)90046-0. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Levison S. W., Ting J. P. Comparison and quantitation of Ia antigen expression on cultured macroglia and ameboid microglia from Lewis rat cerebral cortex: analyses and implications. J Neuroimmunol. 1989 Nov;25(1):63–74. doi: 10.1016/0165-5728(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Nakazato Y. The identity of cells expressing MHC class II antigens in normal and pathological human brain. Neuropathol Appl Neurobiol. 1992 Feb;18(1):13–26. doi: 10.1111/j.1365-2990.1992.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Sethna M. P., Lampson L. A. Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-gamma. J Neuroimmunol. 1991 Nov;34(2-3):121–132. doi: 10.1016/0165-5728(91)90121-m. [DOI] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by an immune interferon-like lymphokine: inhibitory effect of endotoxin. J Immunol. 1982 Dec;129(6):2402–2406. [PubMed] [Google Scholar]
- Steiniger B., van der Meide P. H. Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmunol. 1988 Aug;19(1-2):111–118. doi: 10.1016/0165-5728(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989 Aug;105(2):115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Wentworth P. A., Ziegler H. K. Induction of macrophage Ia expression by lipopolysaccharide and Listeria monocytogenes in congenitally athymic nude mice. J Immunol. 1987 May 15;138(10):3167–3173. [PubMed] [Google Scholar]
- Wikstrand C. J., Bigner D. D. Immunobiologic aspects of the brain and human gliomas. A review. Am J Pathol. 1980 Feb;98(2):517–568. [PMC free article] [PubMed] [Google Scholar]
- Xu J., Kaur C., Ling E. A. Variation with age in the labelling of amoeboid microglial cells in rats following intraperitoneal or intravenous injection of a fluorescent dye. J Anat. 1993 Feb;182(Pt 1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Yem A. W., Parmely M. J. Modulation of Ia-like antigen expression and antigen-presenting activity of human monocytes by endotoxin and zymosan A. J Immunol. 1981 Dec;127(6):2245–2251. [PubMed] [Google Scholar]
- Ziegler H. K., Staffileno L. K., Wentworth P. Modulation of macrophage Ia-expression by lipopolysaccharide. I. Induction of Ia expression in vivo. J Immunol. 1984 Oct;133(4):1825–1835. [PubMed] [Google Scholar]