Abstract
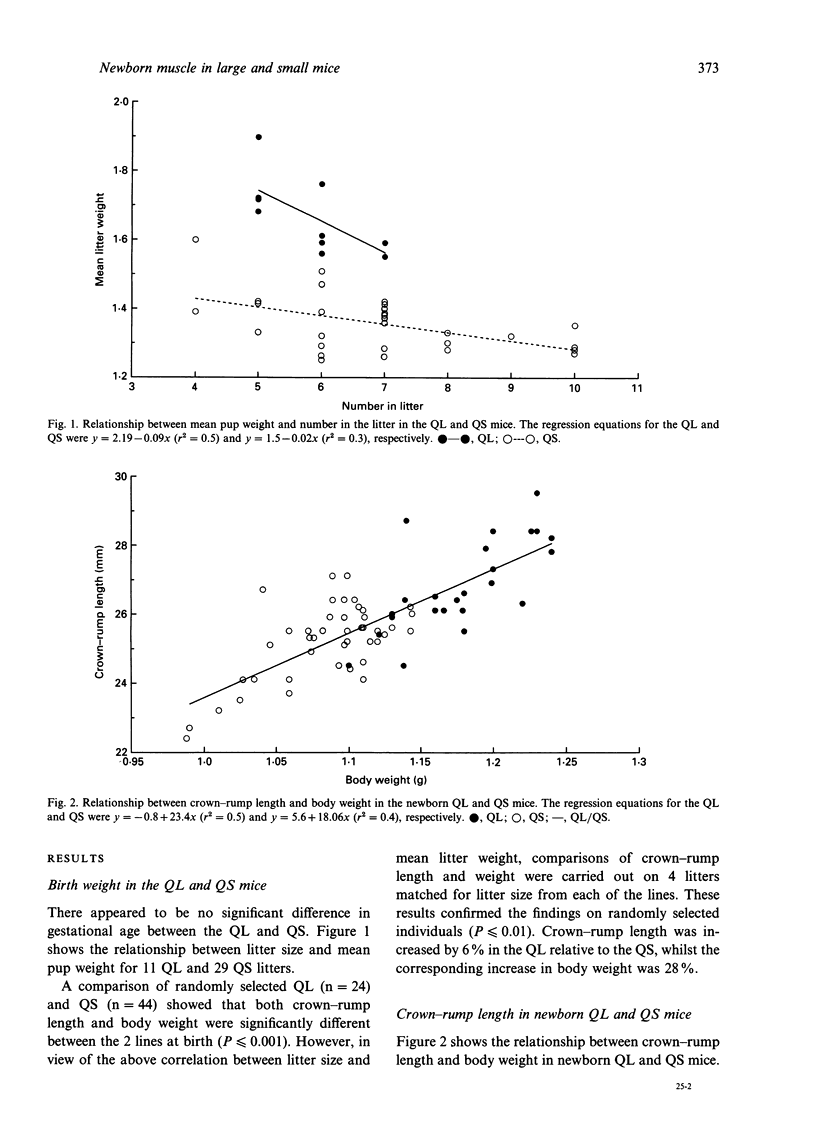
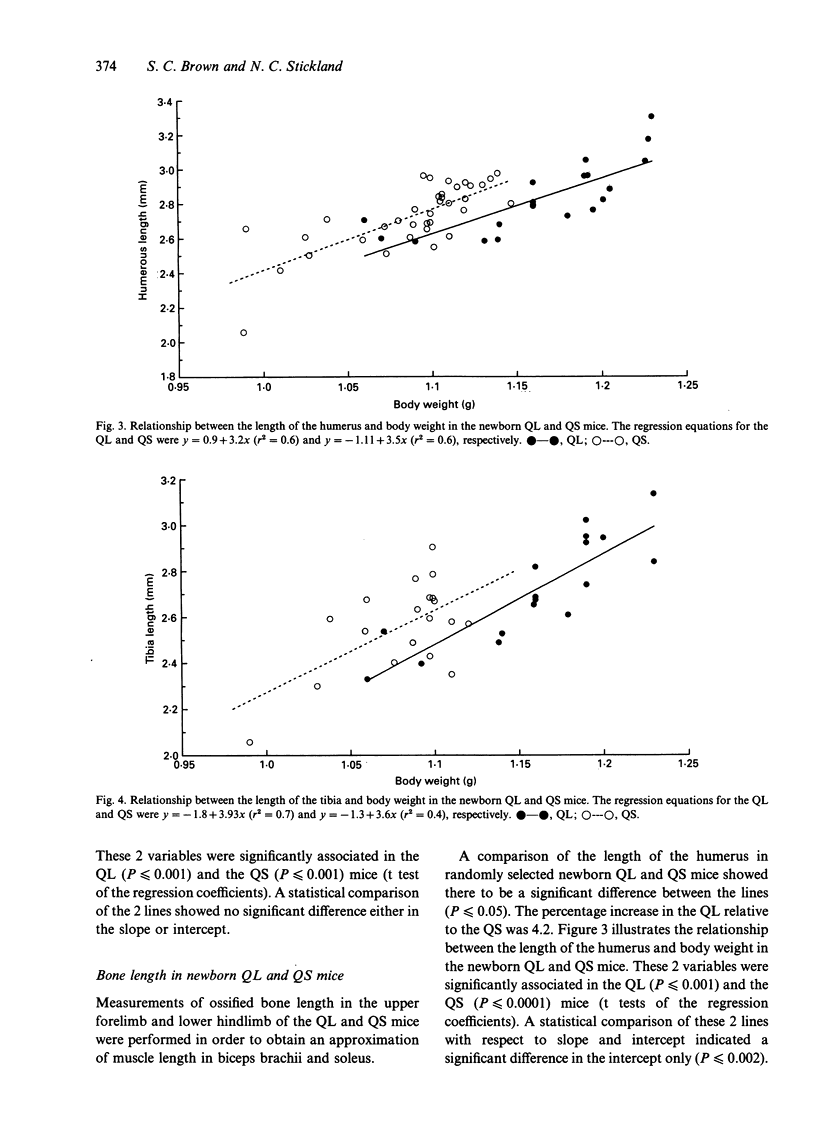
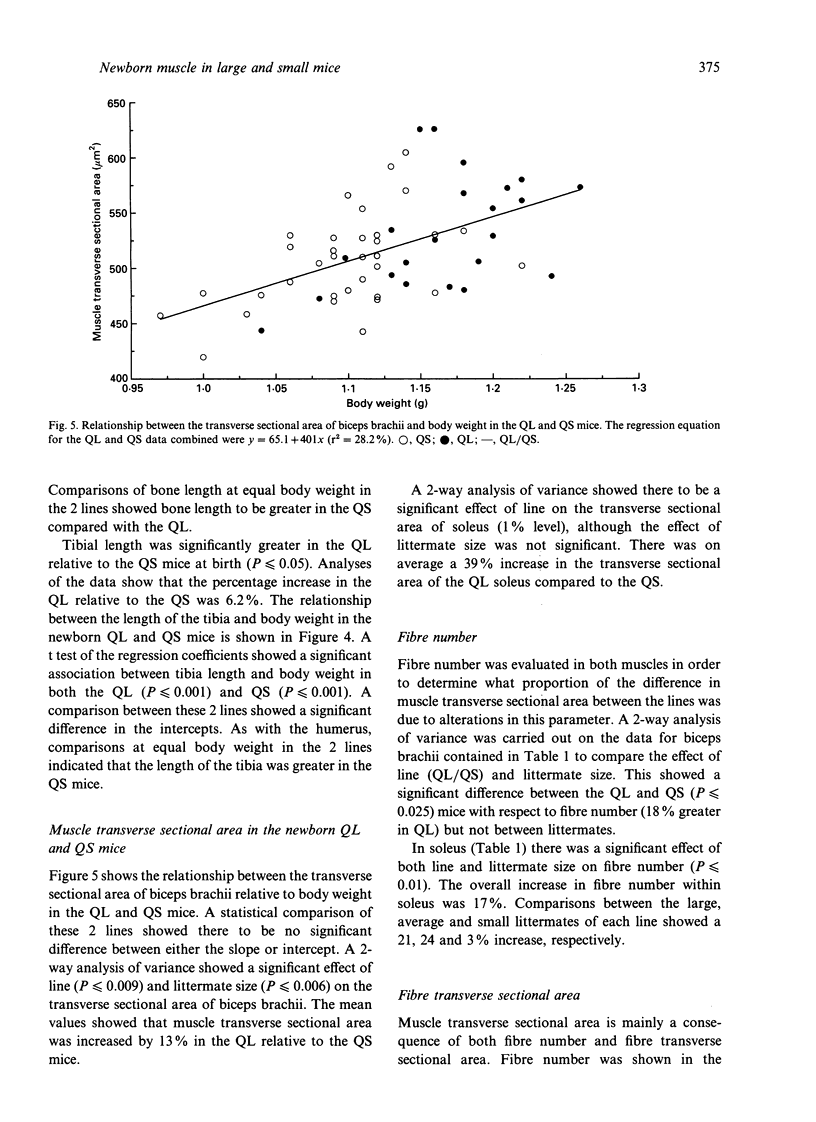
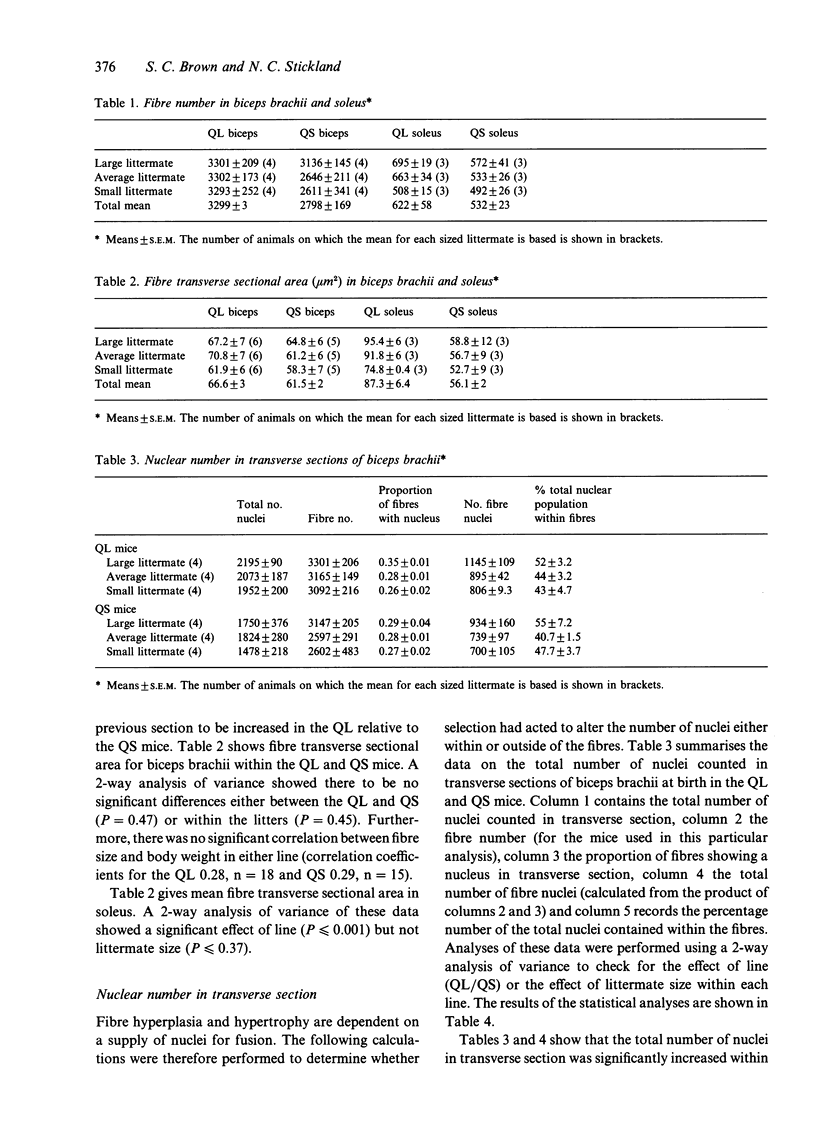
Selection for divergent body weight at 6 wk of age in the Q strain mouse has produced large (QL) and small (QS) mice which differ 2-fold in their adult body weight. The purpose of this investigation was to identify some of the cellular mechanisms which underlie the early divergence in size between the 2 lines. At birth, QL mice (for similar litter sizes) were 28% heavier and 6% longer than QS mice. This was reflected by measurements of longitudinal bone length which were greater in QL (tibia 6.2%, humerus 4.2%) compared with QS mice. Fibre number was found to be 18 and 17% greater in the biceps brachii and soleus muscles respectively of the QL mice. It was concluded that this was not a consequence of any alteration in the ratio of developing secondary to primary myofibres in either muscle. Fibre cross-sectional areas were only significantly different between the QL and QS for the soleus muscle, which might be explained by the relatively greater divergence in the length of its supporting bone (tibia) between the QL and QS compared with the humerus. Estimates of nuclear number showed that there were significantly more nuclei in biceps brachii muscle of QL than in the QS mice which could be attributed to the difference in fibre number, although no such differences were found for the soleus muscle. There was no apparent alteration in the proportion of nuclei found within the fibres of the biceps muscle. Overall the results indicate that selection in this situation has acted through the normal cellular processes of growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguas A. P. The use of osmium tetroxide-potassium ferrocyanide as an extracellular tracer in electron microscopy. Stain Technol. 1982 Mar;57(2):69–73. doi: 10.3109/10520298209066530. [DOI] [PubMed] [Google Scholar]
- Al-Murrani W. K., Roberts R. C. Maternal effects on body weight in mice selected for large and small size. Genet Res. 1978 Nov;32(3):295–302. doi: 10.1017/s0016672300018796. [DOI] [PubMed] [Google Scholar]
- Brown S. C., Stickland N. C. Satellite cell content in muscles of large and small mice. J Anat. 1993 Aug;183(Pt 1):91–96. [PMC free article] [PubMed] [Google Scholar]
- Falconer D. S., Gauld I. K., Roberts R. C. Cell numbers and cell sizes in organs of mice selected for large and small body size. Genet Res. 1978 Jun;31(3):287–301. doi: 10.1017/s0016672300018061. [DOI] [PubMed] [Google Scholar]
- Falconer D. S. Replicated selection for body weight in mice. Genet Res. 1973 Dec;22(3):291–321. doi: 10.1017/s0016672300013094. [DOI] [PubMed] [Google Scholar]
- Gibson M. C., Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983 Oct;6(8):574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- Hooper A. C. Muscles and bones of large and small mice compared at equal body weights. J Anat. 1978 Sep;127(Pt 1):117–123. [PMC free article] [PubMed] [Google Scholar]
- Luff A. R., Goldspink G. Large and small muscles. Life Sci. 1967 Sep 1;6(17):1821–1826. doi: 10.1016/0024-3205(67)90210-x. [DOI] [PubMed] [Google Scholar]
- MCLAREN A. GENETIC AND ENVIRONMENTAL EFFECTS ON FOETAL AND PLACENTAL GROWTH IN MICE. J Reprod Fertil. 1965 Feb;9:79–98. doi: 10.1530/jrf.0.0090079. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Stockdale F. E. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986 Dec;103(6 Pt 1):2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F. P. The relationship between the dimensions of the fibres and the number of nuclei during restricted growth, degrowth and compensatory growth of skeletal muscle. Am J Anat. 1968 May;122(3):565–571. doi: 10.1002/aja.1001220309. [DOI] [PubMed] [Google Scholar]
- Penney R. K., Prentis P. F., Marshall P. A., Goldspink G. Differentiation of muscle and the determination of ultimate tissue size. Cell Tissue Res. 1983;228(2):375–388. doi: 10.1007/BF00204886. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge G. J. Differences in body compositions, growth and food intakes between mice which have been selected for a small and large body size. Br J Nutr. 1981 Nov;46(3):441–450. doi: 10.1079/bjn19810052. [DOI] [PubMed] [Google Scholar]
- Rucklidge G. J. Differences in body compositions, growth and food intakes between mice which have been selected for a small or large body size. Br J Nutr. 1982 Sep;48(2):341–351. doi: 10.1079/bjn19820118. [DOI] [PubMed] [Google Scholar]
- Stickland N. C., Handel S. E. The numbers and types of muscle fibres in large and small breeds of pigs. J Anat. 1986 Aug;147:181–189. [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh H. H., Karlisch P. Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. In Vitro Cell Dev Biol. 1989 Jul;25(7):607–616. doi: 10.1007/BF02623630. [DOI] [PubMed] [Google Scholar]
- Wahlsten D., Bulman-Fleming B. The magnitudes of litter size and sex effects on brain growth of BALB/c mice. Growth. 1987 Summer;51(2):240–248. [PubMed] [Google Scholar]


