Abstract
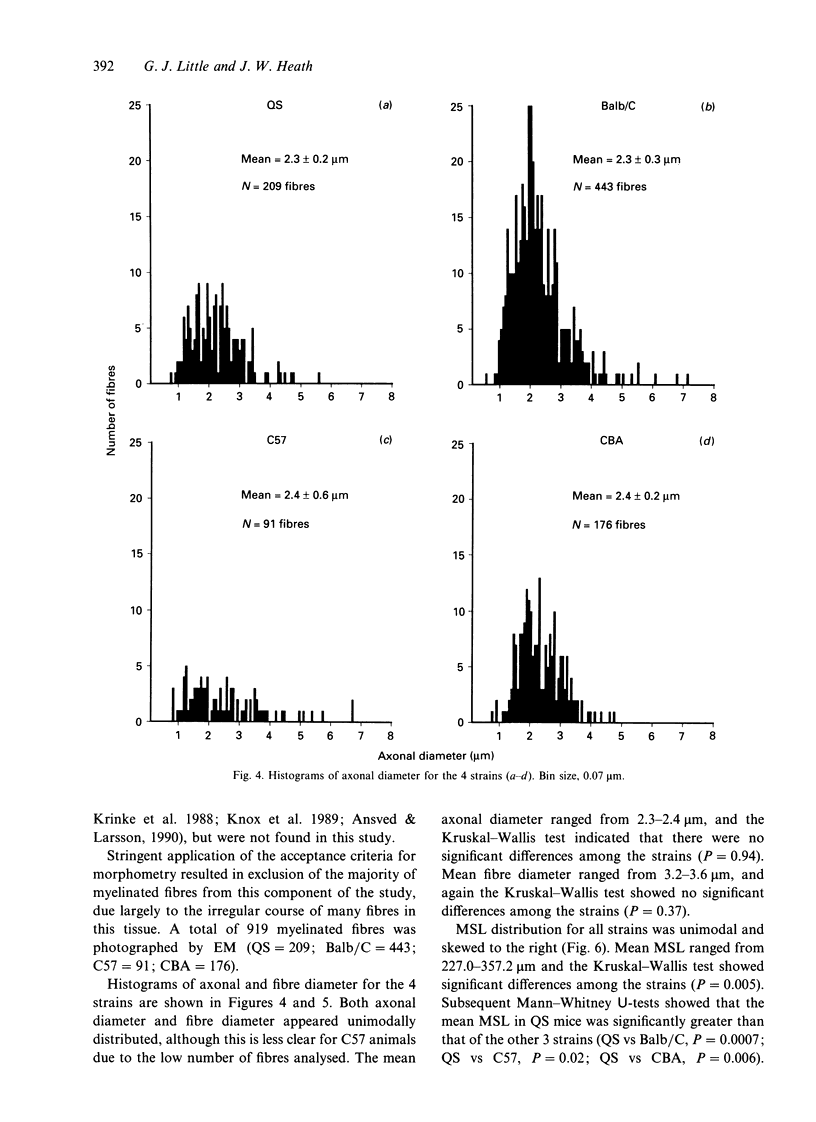
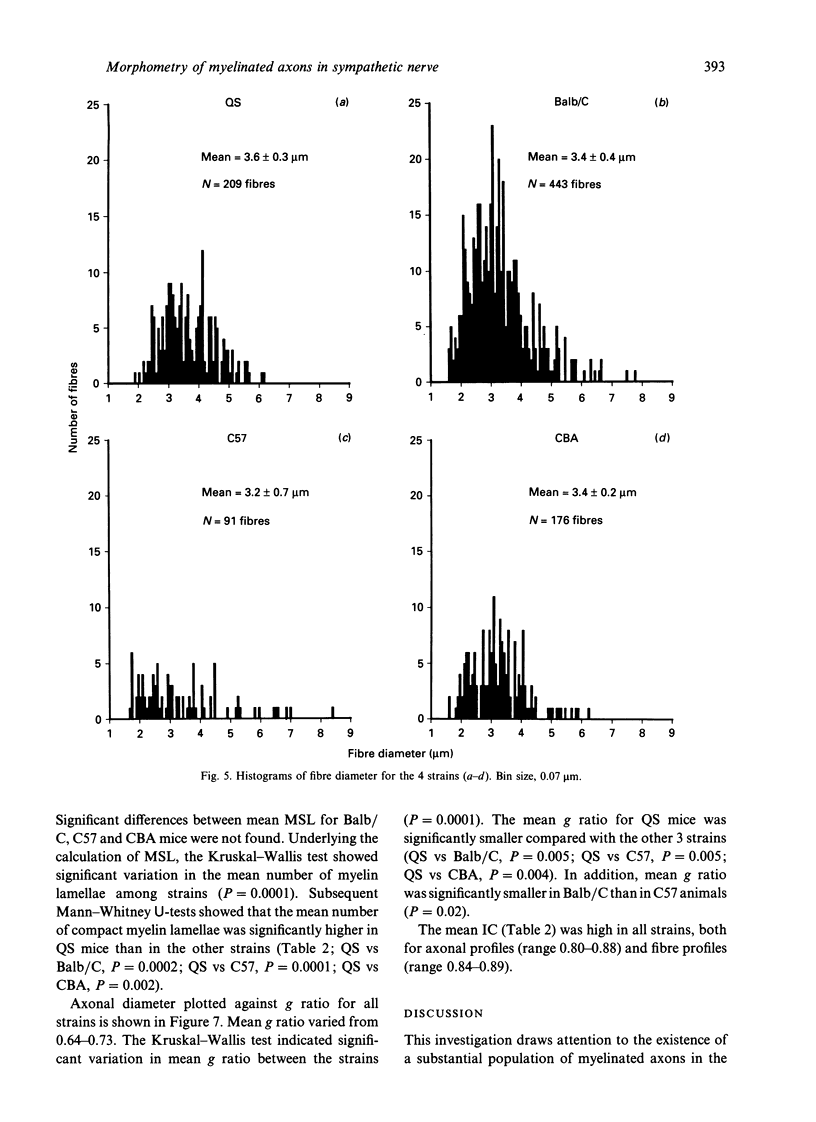
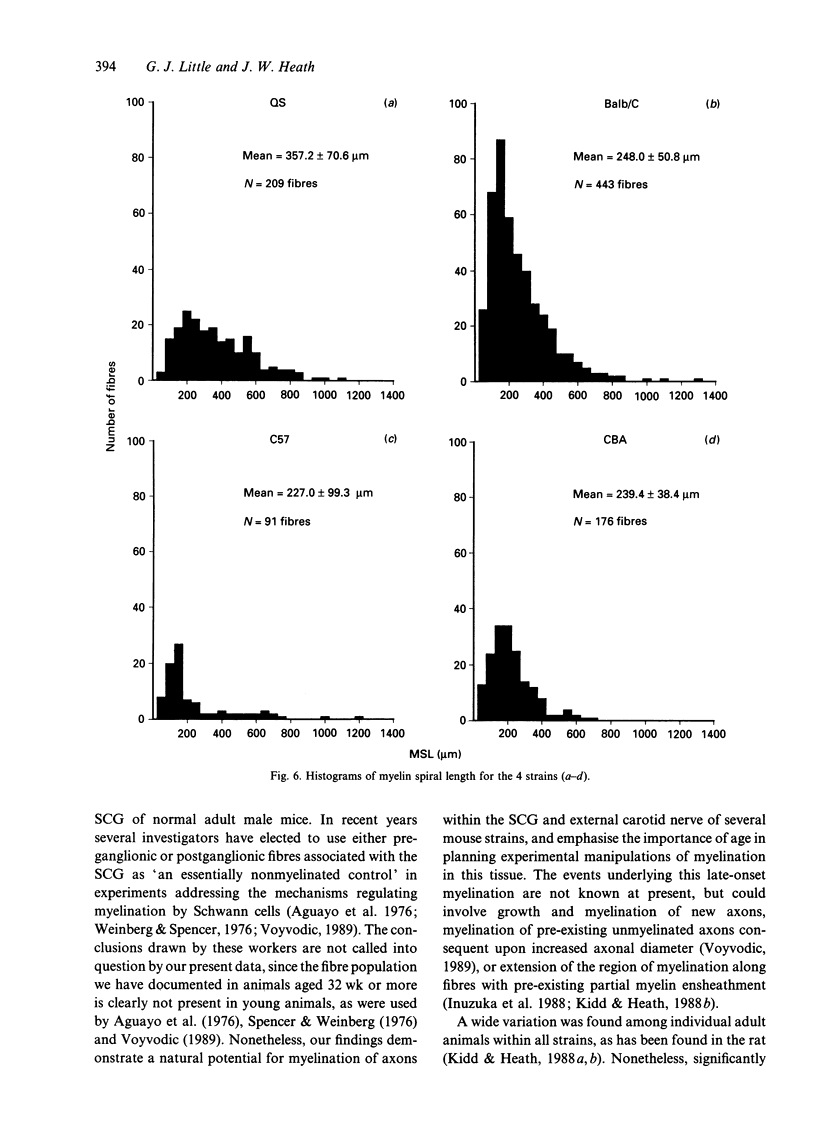
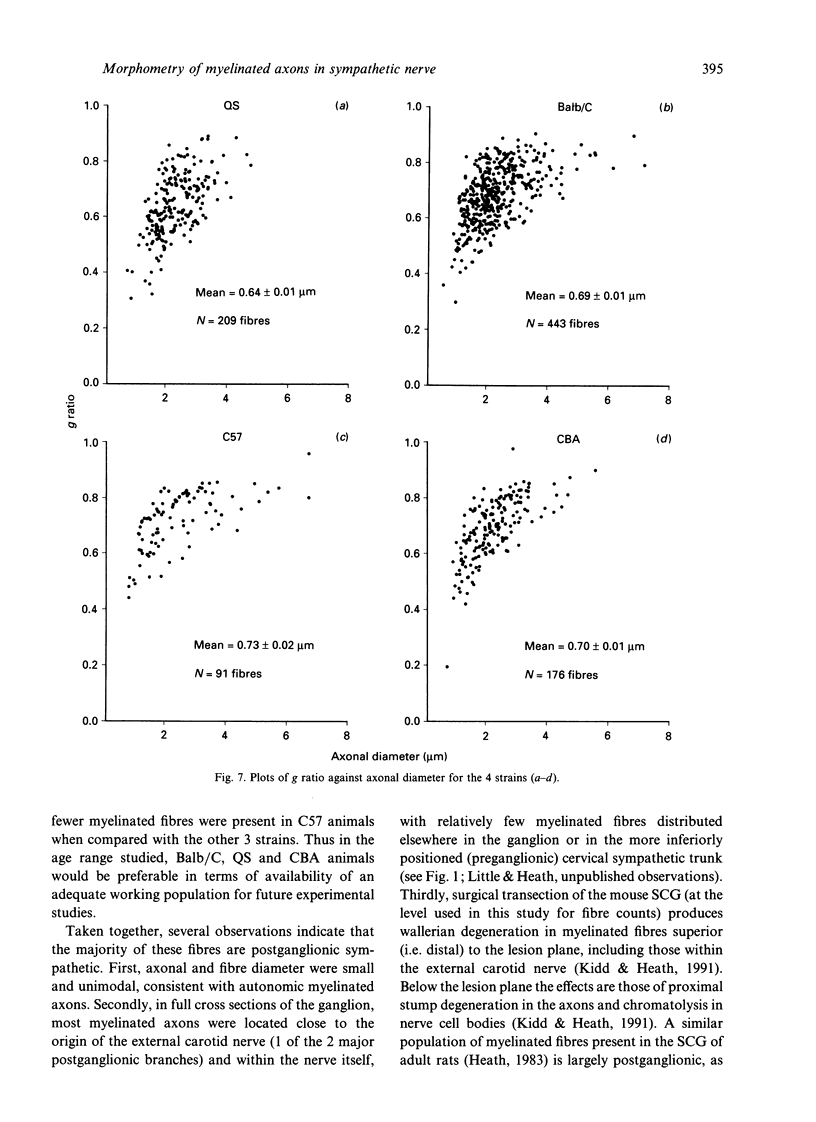
In experimental studies addressing the regulation of myelin formation and maintenance by Schwann cells, the sympathetic nervous system of young adult rodents has served a key role as an essentially nonmyelinated yet modifiable control tissue. Nevertheless there is clear evidence of substantial myelination in the superior cervical ganglion (SCG) of normal mice and rats of more advanced age. Against this background, interpretation of experimental outcomes in particular sympathetic tissues will require detailed quantitative control data taking account of animal age. To provide a baseline for future investigations on myelin remodelling, an ultrastructural morphometric study of myelinated fibres in the SCG was undertaken in 4 strains (QS, Balb/C, C57 and CBA) of adult male mice aged 32-72 wk. Numbers of myelinated fibres in SCG cross-sections varied substantially between individual animals, and the mean numbers for QS (132), Balb/C (165) and CBA (254) were significantly higher than that for C57 (32). Both axonal and fibre diameter were distributed unimodally (means for the 4 strains ranged from 2.3-2.4 microns and 3.2-3.6 microns respectively). Myelin spiral length was distributed unimodally and skewed to the right (range of means = 227-357 microns) and was significantly greater in QS mice as compared with the other 3 strains. While the mean g ratio (axonal diameter/fibre diameter) was significantly lower in QS mice than in the other 3 strains, the range for mean g ratio was 0.64-0.73, indicating that myelination had proceeded appropriately even though late in onset in this tissue. The index of circularity was high in all strains, both for axons (range of means = 0.80-0.88) and fibres (range of means = 0.84-0.89). The small axonal and fibre diameter and unimodal distribution are consistent with the characteristics of autonomic myelinated fibres and it is probable that most are postganglionic sympathetic fibres arising within the SCG. In terms of providing a sufficient population of myelinated fibres for future experimental studies, the QS, Balb/C and CBA strains would be preferable to C57 mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Epps J., Charron L., Bray G. M. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976 Mar 5;104(1):1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- Ansved T., Larsson L. Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. Relation to the number of soleus muscle fibers. J Neurol Sci. 1990 May;96(2-3):269–282. doi: 10.1016/0022-510x(90)90138-d. [DOI] [PubMed] [Google Scholar]
- Appenzeller O., Ogin G. Myelinated fibres in the human paravertebral sympathetic chain; quantitative studies on white rami communicantes. J Neurol Neurosurg Psychiatry. 1973 Oct;36(5):777–785. doi: 10.1136/jnnp.36.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott E. R., Ballard K. J., Boyd I. A., Kalu K. U. Quantitative study of the non-circularity of myelinated peripheral nerve fibres in the cat. J Physiol. 1980 Nov;308:99–123. doi: 10.1113/jphysiol.1980.sp013464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R. T., Bishop Y., Hedley-Whyte E. T. A contribution to the electron microscopic morphometric analysis of peripheral nerve. J Comp Neurol. 1978 Mar 1;178(1):177–186. doi: 10.1002/cne.901780110. [DOI] [PubMed] [Google Scholar]
- Cavaletti G., Tredici G., Marmiroli P., Petruccioli M. G., Barajon I., Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol. 1992;84(4):364–371. doi: 10.1007/BF00227662. [DOI] [PubMed] [Google Scholar]
- Dockery P., Sharma A. K. Ultrastructural abnormalities of myelinated fibres in the tibial nerve of streptozotocin-diabetic rats. J Neurol Sci. 1990 Sep;98(2-3):327–345. doi: 10.1016/0022-510x(90)90273-p. [DOI] [PubMed] [Google Scholar]
- Fraher J. P., Kaar G. F., Bristol D. C., Rossiter J. P. Development of ventral spinal motoneurone fibres: a correlative study of the growth and maturation of central and peripheral segments of large and small fibre classes. Prog Neurobiol. 1988;31(3):199–239. doi: 10.1016/0301-0082(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Fraher J. P., O'Leary D., Moran M. A., Cole M., King R. H., Thomas P. K. Relative growth and maturation of axon size and myelin thickness in the tibial nerve of the rat. 1. Normal animals. Acta Neuropathol. 1990;79(4):364–374. doi: 10.1007/BF00308712. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Beuche W. A new approach toward analyzing peripheral nerve fiber populations. I. Variance in sheath thickness corresponds to different geometric proportions of the internodes. J Neuropathol Exp Neurol. 1985 Jan;44(1):60–72. doi: 10.1097/00005072-198501000-00005. [DOI] [PubMed] [Google Scholar]
- Friede R. L., Beuche W. Combined scatter diagrams of sheath thickness and fibre calibre in human sural nerves: changes with age and neuropathy. J Neurol Neurosurg Psychiatry. 1985 Aug;48(8):749–756. doi: 10.1136/jnnp.48.8.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Bischhausen R. The precise geometry of large internodes. J Neurol Sci. 1980 Dec;48(3):367–381. doi: 10.1016/0022-510x(80)90109-4. [DOI] [PubMed] [Google Scholar]
- Gillespie M. J., Stein R. B. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983 Jan 17;259(1):41–56. doi: 10.1016/0006-8993(83)91065-x. [DOI] [PubMed] [Google Scholar]
- Guy J., Ellis E. A., Kelley K., Hope G. M. Spectra of G ratio, myelin sheath thickness, and axon and fiber diameter in the guinea pig optic nerve. J Comp Neurol. 1989 Sep 22;287(4):446–454. doi: 10.1002/cne.902870404. [DOI] [PubMed] [Google Scholar]
- Heath J. W. Double myelination of axons in the sympathetic nervous system. J Neurocytol. 1982 Apr;11(2):249–262. doi: 10.1007/BF01258246. [DOI] [PubMed] [Google Scholar]
- Heath J. W., Kidd G. J., Trapp B. D., Dunkley P. R. Myelin maintenance by Schwann cells in the absence of axons. Neurosci Lett. 1991 Jul 22;128(2):277–280. doi: 10.1016/0304-3940(91)90279-3. [DOI] [PubMed] [Google Scholar]
- Inuzuka T., Quarles R. H., Trapp B. D., Heath J. W. Analysis of myelin proteins in sympathetic peripheral nerve of adult rats. Brain Res. 1988 Feb 1;466(2):191–199. doi: 10.1016/0165-3806(88)90044-2. [DOI] [PubMed] [Google Scholar]
- Karnes J., Robb R., O'Brien P. C., Lambert E. H., Dyck P. J. Computerized image recognition for morphometry of nerve attribute of shape of sampled transverse sections of myelinated fibers which best estimates their average diameter. J Neurol Sci. 1977 Oct;34(1):43–51. doi: 10.1016/0022-510x(77)90090-9. [DOI] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W. Double myelination of axons in the sympathetic nervous system of the mouse. I. Ultrastructural features and distribution. J Neurocytol. 1988 Apr;17(2):245–261. doi: 10.1007/BF01674211. [DOI] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W. Double myelination of axons in the sympathetic nervous system of the mouse. II. Mechanisms of formation. J Neurocytol. 1988 Apr;17(2):263–276. doi: 10.1007/BF01674212. [DOI] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W., Dunkley P. R. Degeneration of myelinated sympathetic nerve fibres following treatment with guanethidine. J Neurocytol. 1986 Oct;15(5):561–572. doi: 10.1007/BF01611857. [DOI] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W. Myelin sheath survival following axonal degeneration in doubly myelinated nerve fibers. J Neurosci. 1991 Dec;11(12):4003–4014. doi: 10.1523/JNEUROSCI.11-12-04003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G. J., Heath J. W., Trapp B. D., Dunkley P. R. Myelin sheath survival after guanethidine-induced axonal degeneration. J Cell Biol. 1992 Jan;116(2):395–403. doi: 10.1083/jcb.116.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. A., Kokmen E., Dyck P. J. Morphometric alteration of rat myelinated fibers with aging. J Neuropathol Exp Neurol. 1989 Mar;48(2):119–139. doi: 10.1097/00005072-198903000-00001. [DOI] [PubMed] [Google Scholar]
- Krinke G., Froehlich E., Herrmann M., Schnider K., Da Silva F., Suter J., Traber K. Adjustment of the myelin sheath to axonal atrophy in the rat spinal root by the formation of infolded myelin loops. Acta Anat (Basel) 1988;131(3):182–187. doi: 10.1159/000146510. [DOI] [PubMed] [Google Scholar]
- Low P. A., Walsh J. C., Huang C. Y., McLeod J. G. The sympathetic nervous system in alcoholic neuropathy. A clinical and pathological study. Brain. 1975 Sep;98(3):357–364. doi: 10.1093/brain/98.3.357. [DOI] [PubMed] [Google Scholar]
- Low P. A., Walsh J. C., Huang C. Y., McLeod J. G. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain. 1975 Sep;98(3):341–356. doi: 10.1093/brain/98.3.341. [DOI] [PubMed] [Google Scholar]
- O'Neill J. H., Gilliatt R. W. Adaptation of the myelin sheath during axonal atrophy. Acta Neuropathol. 1987;74(1):62–66. doi: 10.1007/BF00688339. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Bohl J., Brodda K. Changes of the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol. 1978 Aug 7;43(1-2):169–178. doi: 10.1007/BF00685012. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Koles Z. J. Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. Am J Physiol. 1970 Nov;219(5):1256–1258. doi: 10.1152/ajplegacy.1970.219.5.1256. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Raine C. S., Wiśniewski H. Axon diameter and myelin thickness. Unusual relationships in dorsal root ganglia. Anat Rec. 1973 Jun;176(2):225–243. doi: 10.1002/ar.1091760209. [DOI] [PubMed] [Google Scholar]
- Tuisku F., Hildebrand C. Nodes of Ranvier and myelin sheath dimensions along exceptionally thin myelinated vertebrate PNS axons. J Neurocytol. 1992 Nov;21(11):796–806. doi: 10.1007/BF01237905. [DOI] [PubMed] [Google Scholar]
- Voyvodic J. T. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989 Nov 23;342(6248):430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976 Aug 27;113(2):363–378. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]
- Wheeler S. J., Plummer J. M. Age-related changes in the fibre composition of equine peripheral nerve. J Neurol Sci. 1989 Mar;90(1):53–66. doi: 10.1016/0022-510x(89)90045-2. [DOI] [PubMed] [Google Scholar]
- Williams P. L., Wendell-Smith C. P. Some additional parametric variations between peripheral nerve fibre populations. J Anat. 1971 Sep;109(Pt 3):505–526. [PMC free article] [PubMed] [Google Scholar]