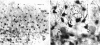Abstract
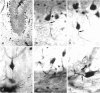
The murine cerebellum was investigated by light microscopy using an improved modification of Ehrlich's methylene blue supravital staining technique. The dye exhibited a special affinity for the perikarya as well as the axons of Purkinje cells. In addition, large fusiform or stellate nerve cells which were characterised by long descending axons were seen to be distributed diffusely within the granular layer and the subcortical white matter. These findings indicate the existence of a 2nd type of projection neuron besides the Purkinje cells and are therefore in full accordance with older neuroanatomical observations based on silver impregnation. When correlated with recent studies on the occurrence of different calcium-binding proteins, the results show that the large perikarya demonstrated immunohistochemically within the granular layer seem to belong to the group of methylene blue positive neurons. Nevertheless, the definitive association of a single neuron with a nerve cell class is only possible if the axon is stained and clearly identifiable. Because of its selectivity for a special type of nerve cell, including its axon, the histological method used in this study may therefore also be suitable for investigating other parts of the brain and the spinal cord.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso J. R., Arèvalo R., Briñn J. G., Lara J., Weruaga E., Aijòn J. Parvalbumin immunoreactive neurons and fibres in the teleost cerebellum. Anat Embryol (Berl) 1992;185(4):355–361. doi: 10.1007/BF00188547. [DOI] [PubMed] [Google Scholar]
- Braak E., Braak H. On three types of large nerve cells in the granular layer of the human cerebellar cortex. Anat Embryol (Berl) 1983;166(1):67–86. doi: 10.1007/BF00317945. [DOI] [PubMed] [Google Scholar]
- Braak H. On the intermediate cells of lugaro within the cerebellar cortex of man. A pigmentarchitectonic study. Cell Tissue Res. 1974 Jun 12;149(3):399–411. doi: 10.1007/BF00226773. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Fournet N., Garcia-Segura L. M., Norman A. W., Orci L. Selective localization of calcium-binding protein in human brainstem, cerebellum and spinal cord. Brain Res. 1986 Dec 10;399(2):310–316. doi: 10.1016/0006-8993(86)91521-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L. M., Baetens D., Roth J., Norman A. W., Orci L. Immunohistochemical mapping of calcium-binding protein immunoreactivity in the rat central nervous system. Brain Res. 1984 Mar 26;296(1):75–86. doi: 10.1016/0006-8993(84)90512-2. [DOI] [PubMed] [Google Scholar]
- Müller T. Light-microscopic demonstration of methylene blue accumulation sites in mouse brain after supravital staining. Acta Anat (Basel) 1992;144(1):39–44. [PubMed] [Google Scholar]
- O'Leary J. L., Petty J., Harris A. B., Inukai J. Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol. 1968 Jul;43(4):197–201. doi: 10.3109/10520296809115068. [DOI] [PubMed] [Google Scholar]
- Scotti A. L., Nitsch C. Differential Ca2+ binding properties in the human cerebellar cortex: distribution of parvalbumin and calbindin D-28k immunoreactivity. Anat Embryol (Berl) 1992;185(2):163–167. doi: 10.1007/BF00185917. [DOI] [PubMed] [Google Scholar]
- Stichel C. C., Kägi U., Heizmann C. W. Parvalbumin in cat brain: isolation, characterization, and localization. J Neurochem. 1986 Jul;47(1):46–53. doi: 10.1111/j.1471-4159.1986.tb02829.x. [DOI] [PubMed] [Google Scholar]