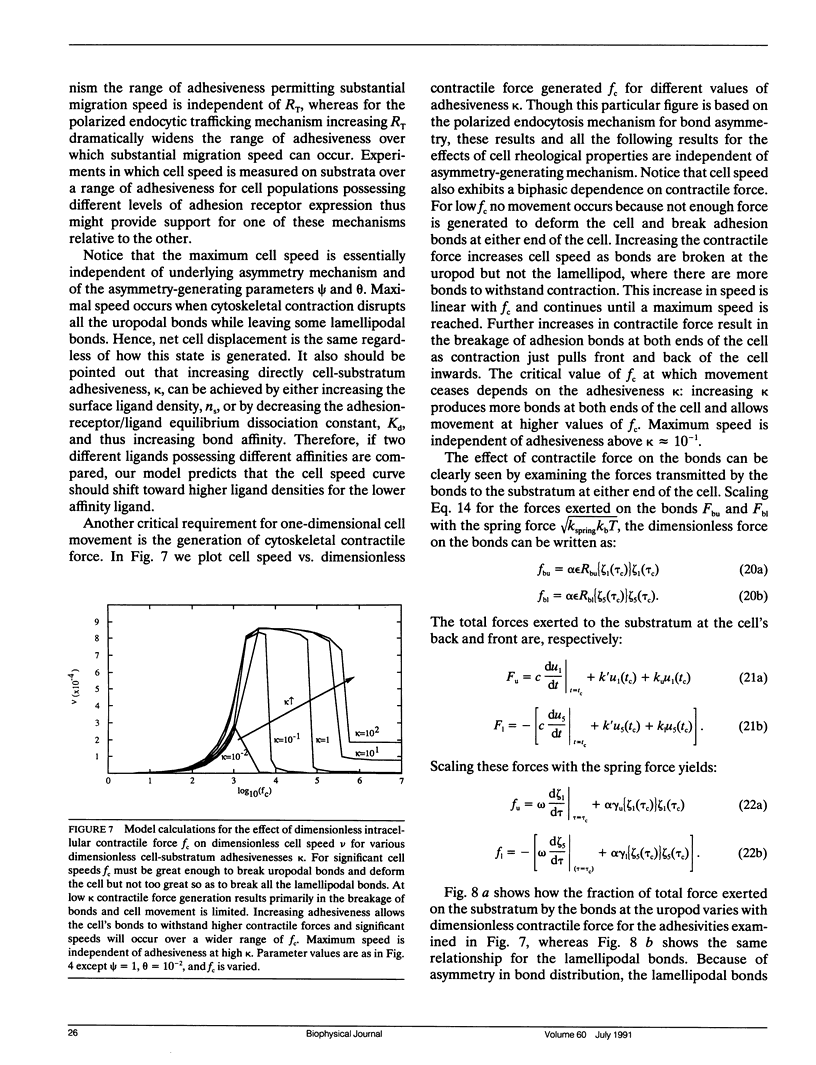
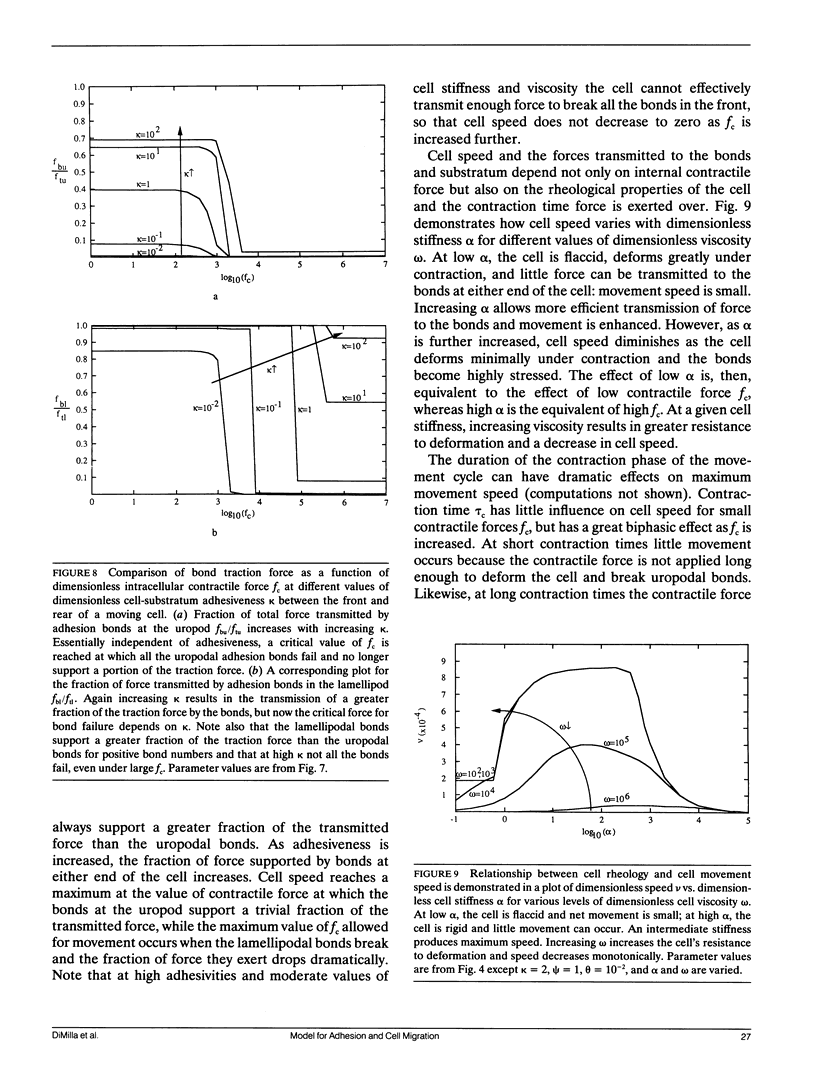
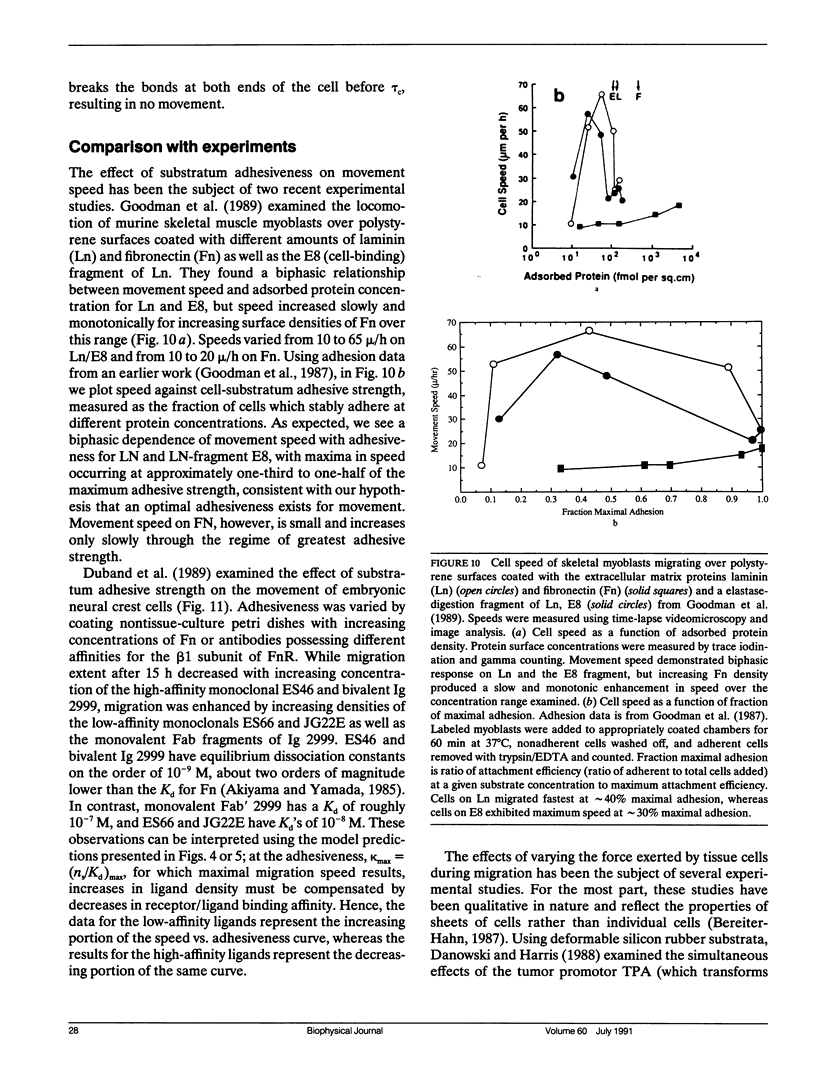
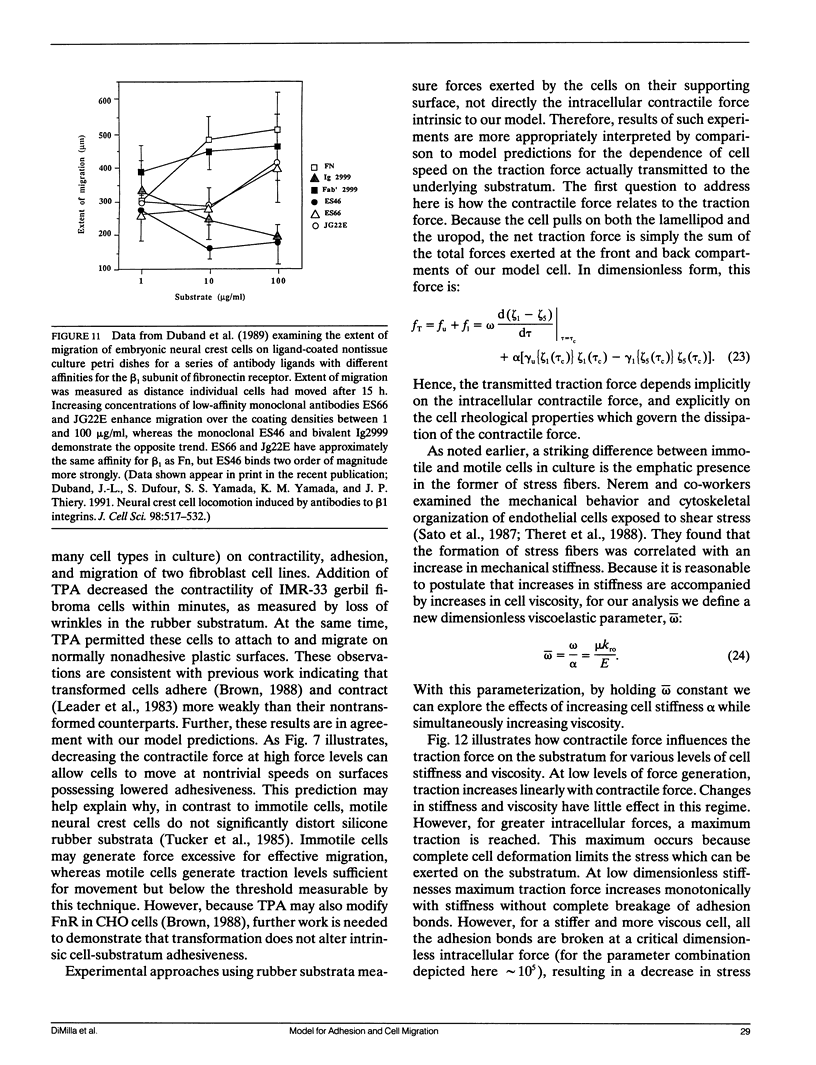
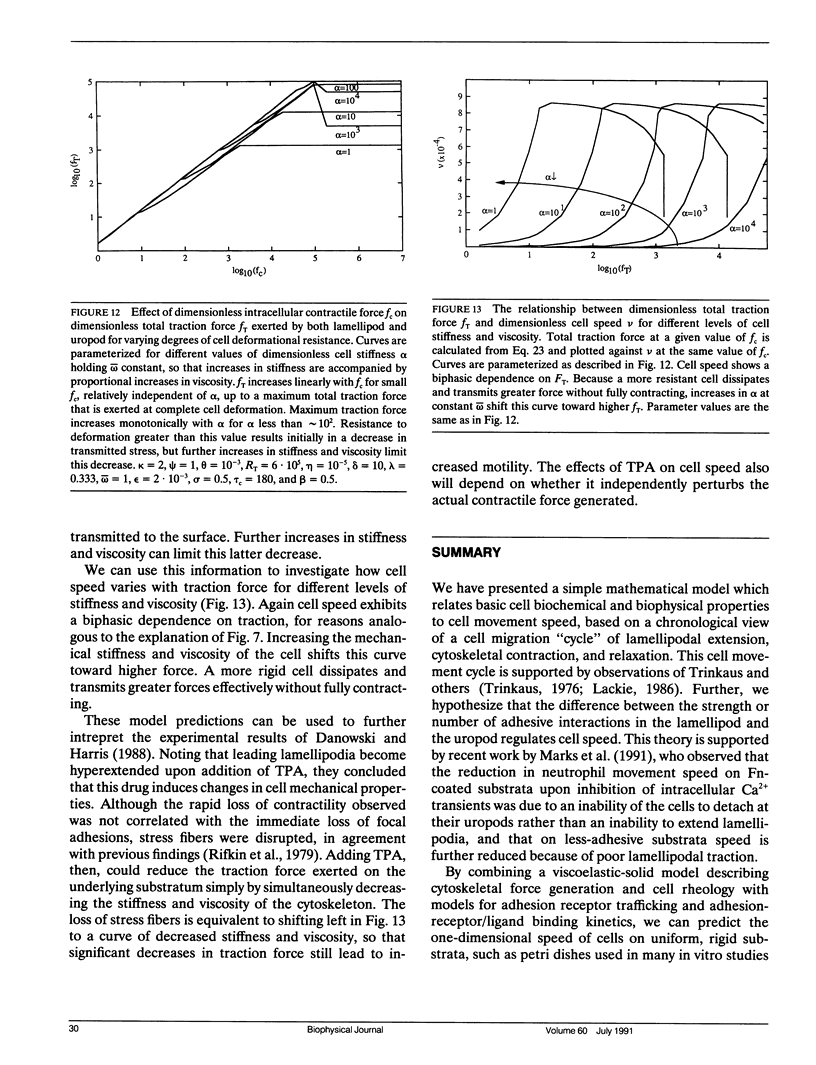
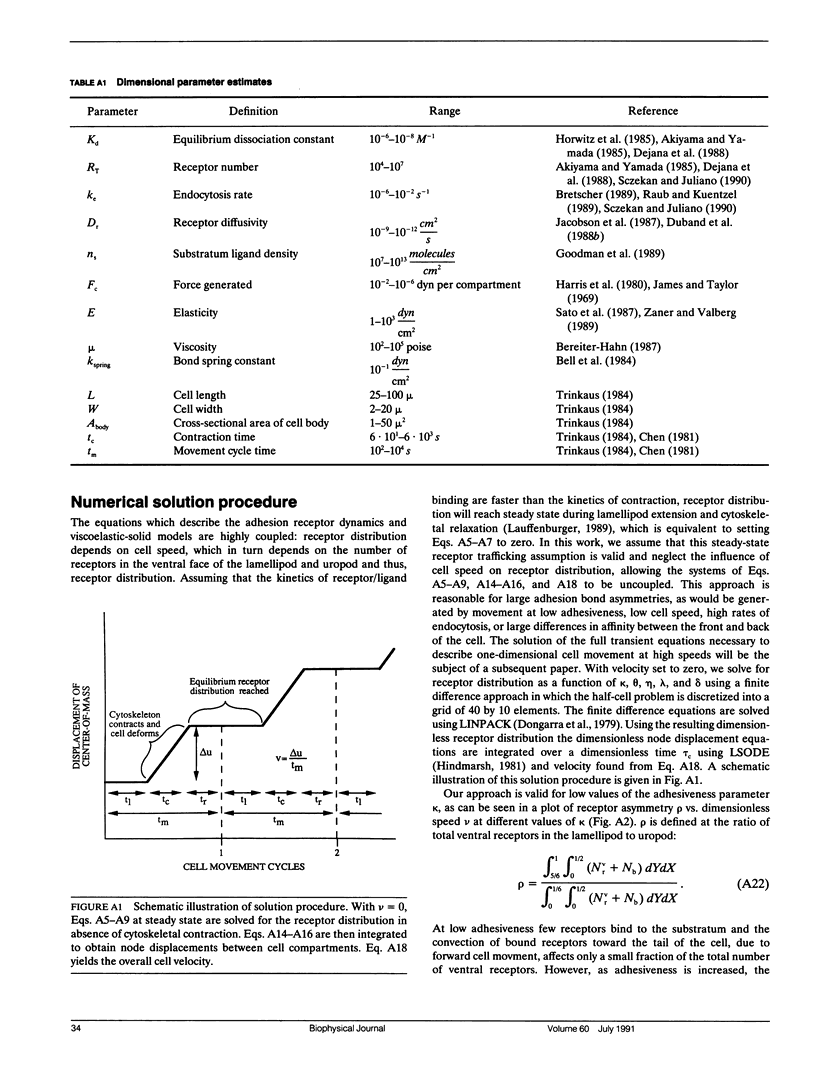
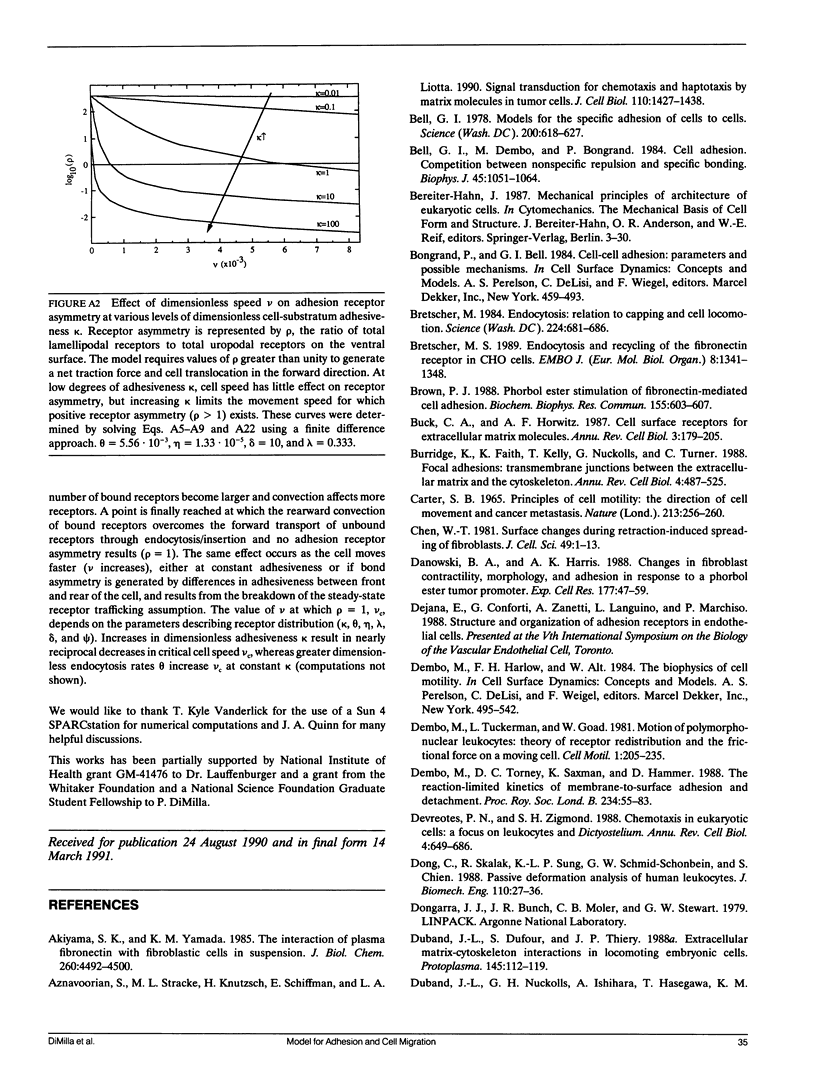
Abstract
Migration of mammalian blood and tissue cells over adhesive surfaces is apparently mediated by specific reversible reactions between cell membrane adhesion receptors and complementary ligands attached to the substratum. Although in a number of systems these receptors and ligand molecules have been isolated and identified, a theory capable of predicting the effects of their properties on cell migration behavior currently does not exist. We present a simple mathematical model for elucidating the dependence of cell speed on adhesion-receptor/ligand binding and cell mechanical properties. Our model can be applied to propose answers to questions such as: does an optimal adhesiveness exist for cell movement? How might changes in receptor and ligand density and/or affinity affect the rate of migration? Can cell rheological properties influence movement speed? This model incorporates cytoskeletal force generation, cell polarization, and dynamic adhesion as requirements for persistent cell movement. A critical feature is the proposed existence of an asymmetry in some cell adhesion-receptor property, correlated with cell polarity. We consider two major alternative mechanisms underlying this asymmetry: (a) a spatial distribution of adhesion-receptor number due to polarized endocytic trafficking and (b) a spatial variation in adhesion-receptor/ligand bond strength. Applying a viscoelastic-solid model for cell mechanics allows us to represent one-dimensional locomotion with a system of differential equations describing cell deformation and displacement along with adhesion-receptor dynamics. In this paper, we solve these equations under the simplifying assumption that receptor dynamics are at a quasi-steady state relative to cell locomotion. Thus, our results are strictly valid for sufficiently slow cell movement, as typically observed for tissue cells such as fibroblasts. Numerical examples relevant to experimental systems are provided. Our results predict how cell speed might vary with intracellular contractile force, cell rheology, receptor/ligand kinetics, and receptor/ligand number densities. A biphasic dependence is shown to be possible with respect to some of the system parameters, with position of the maxima essentially governed by a balance between transmitted contractile force and adhesiveness. We demonstrate that predictions for the two alternative asymmetry mechanisms can be distinguished and could be experimentally tested using cell populations possessing different adhesion-receptor numbers.
Full text
PDF



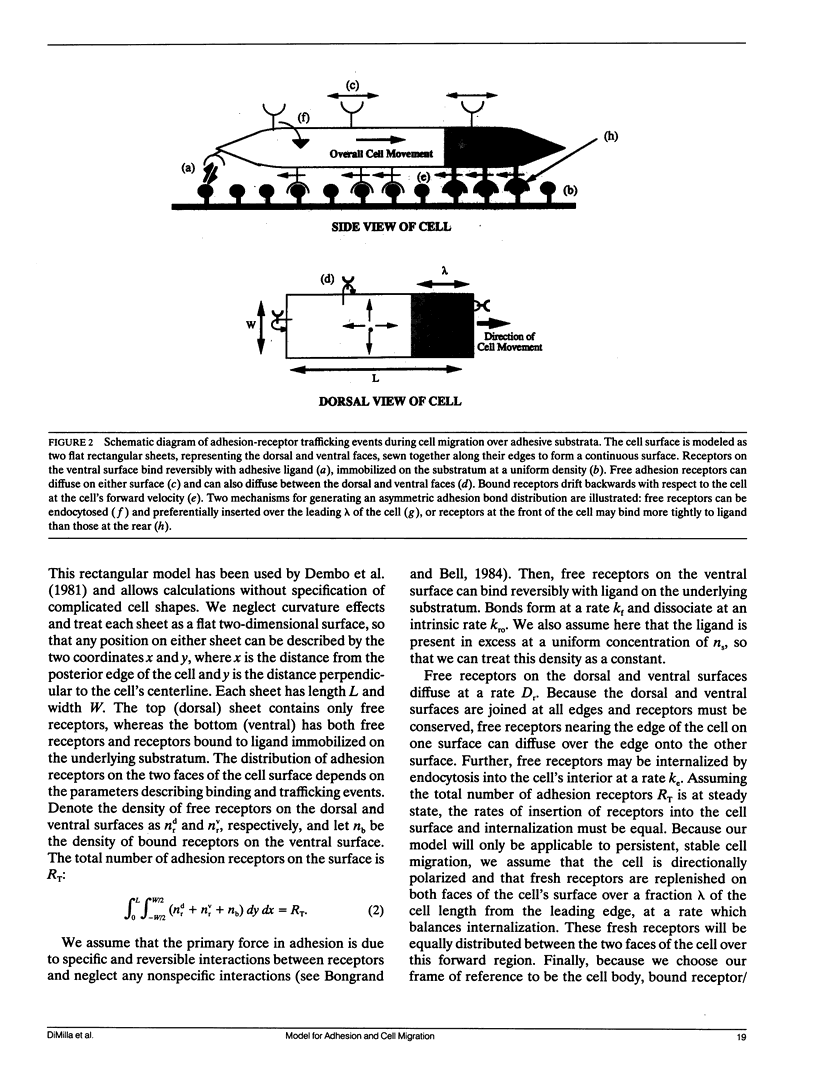
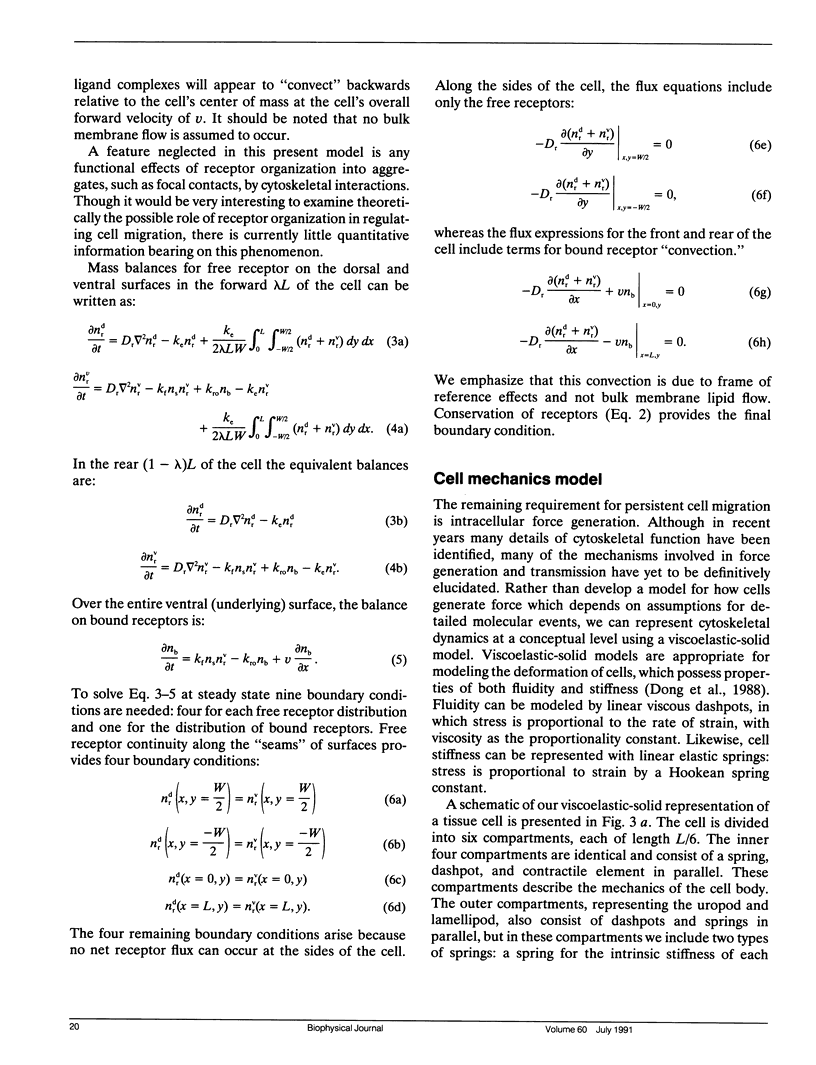






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Aznavoorian S., Stracke M. L., Krutzsch H., Schiffmann E., Liotta L. A. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990 Apr;110(4):1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Dembo M., Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984 Jun;45(6):1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I. Models for the specific adhesion of cells to cells. Science. 1978 May 12;200(4342):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989 May;8(5):1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis: relation to capping and cell locomotion. Science. 1984 May 18;224(4650):681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- Brown P. J. Phorbol ester stimulation of fibronectin-mediated cell adhesion. Biochem Biophys Res Commun. 1988 Sep 15;155(2):603–607. doi: 10.1016/s0006-291x(88)80537-0. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chen W. T. Surface changes during retraction-induced spreading of fibroblasts. J Cell Sci. 1981 Jun;49:1–13. doi: 10.1242/jcs.49.1.1a. [DOI] [PubMed] [Google Scholar]
- Danowski B. A., Harris A. K. Changes in fibroblast contractility, morphology, and adhesion in response to a phorbol ester tumor promoter. Exp Cell Res. 1988 Jul;177(1):47–59. doi: 10.1016/0014-4827(88)90024-9. [DOI] [PubMed] [Google Scholar]
- Dembo M., Torney D. C., Saxman K., Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B Biol Sci. 1988 Jun 22;234(1274):55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- Dembo M., Tuckerman L., Goad W. Motion of polymorphonuclear leukocytes: theory of receptor redistribution and the frictional force on a moving cell. Cell Motil. 1981;1(2):205–235. doi: 10.1002/cm.970010205. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Dong C., Skalak R., Sung K. L., Schmid-Schönbein G. W., Chien S. Passive deformation analysis of human leukocytes. J Biomech Eng. 1988 Feb;110(1):27–36. doi: 10.1115/1.3108402. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Nuckolls G. H., Ishihara A., Hasegawa T., Yamada K. M., Thiery J. P., Jacobson K. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988 Oct;107(4):1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J., Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988 Sep 15;263(26):12927–12932. [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Deutzmann R., von der Mark K. Two distinct cell-binding domains in laminin can independently promote nonneuronal cell adhesion and spreading. J Cell Biol. 1987 Jul;105(1):589–598. doi: 10.1083/jcb.105.1.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. L., Risse G., von der Mark K. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol. 1989 Aug;109(2):799–809. doi: 10.1083/jcb.109.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Focal adhesion sites and the removal of substratum-bound fibronectin. J Cell Biol. 1986 Dec;103(6 Pt 2):2697–2706. doi: 10.1083/jcb.103.6.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K., Wild P., Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980 Apr 11;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Harris A. Behavior of cultured cells on substrata of variable adhesiveness. Exp Cell Res. 1973 Mar 15;77(1):285–297. doi: 10.1016/0014-4827(73)90579-x. [DOI] [PubMed] [Google Scholar]
- Heath J. P. Behaviour and structure of the leading lamella in moving fibroblasts. I. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell surface. J Cell Sci. 1983 Mar;60:331–354. doi: 10.1242/jcs.60.1.331. [DOI] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Holifield B., Jacobson K. Analysis of lateral redistribution of a plasma membrane glycoprotein-monoclonal antibody complex [corrected]. J Cell Biol. 1988 Feb;106(2):329–343. doi: 10.1083/jcb.106.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Ishihara A., Inman R. Lateral diffusion of proteins in membranes. Annu Rev Physiol. 1987;49:163–175. doi: 10.1146/annurev.ph.49.030187.001115. [DOI] [PubMed] [Google Scholar]
- James D. W., Taylor J. F. The stress developed by sheets of chick fibroblasts in vitro. Exp Cell Res. 1969 Jan;54(1):107–110. doi: 10.1016/0014-4827(69)90299-7. [DOI] [PubMed] [Google Scholar]
- Leader W. M., Stopak D., Harris A. K. Increased contractile strength and tightened adhesions to the substratum result from reverse transformation of CHO cells by dibutyryl cyclic adenosine monophosphate. J Cell Sci. 1983 Nov;64:1–11. doi: 10.1242/jcs.64.1.1. [DOI] [PubMed] [Google Scholar]
- Lee J., Gustafsson M., Magnusson K. E., Jacobson K. The direction of membrane lipid flow in locomoting polymorphonuclear leukocytes. Science. 1990 Mar 9;247(4947):1229–1233. doi: 10.1126/science.2315695. [DOI] [PubMed] [Google Scholar]
- Marks P. W., Hendey B., Maxfield F. R. Attachment to fibronectin or vitronectin makes human neutrophil migration sensitive to alterations in cytosolic free calcium concentration. J Cell Biol. 1991 Jan;112(1):149–158. doi: 10.1083/jcb.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Kelly T., Dai M. Z., Dai H. N., Chen W. T. Dynamic cytoskeleton-integrin associations induced by cell binding to immobilized fibronectin. J Cell Biol. 1989 Dec;109(6 Pt 2):3455–3464. doi: 10.1083/jcb.109.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster G. F., Perelson A. S. The physics of cell motility. J Cell Sci Suppl. 1987;8:35–54. doi: 10.1242/jcs.1987.supplement_8.3. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., McConnaughey W. B., Elson E. L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub T. J., Kuentzel S. L. Kinetic and morphological evidence for endocytosis of mammalian cell integrin receptors by using an anti-fibronectin receptor beta subunit monoclonal antibody. Exp Cell Res. 1989 Oct;184(2):407–426. doi: 10.1016/0014-4827(89)90340-6. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sato M., Levesque M. J., Nerem R. M. Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Arteriosclerosis. 1987 May-Jun;7(3):276–286. doi: 10.1161/01.atv.7.3.276. [DOI] [PubMed] [Google Scholar]
- Sato M., Schwarz W. H., Pollard T. D. Dependence of the mechanical properties of actin/alpha-actinin gels on deformation rate. 1987 Feb 26-Mar 4Nature. 325(6107):828–830. doi: 10.1038/325828a0. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner C. L., Bauer J. S., Danilov Y. N., Hussein S., Sczekan M. M., Juliano R. L. Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibronectin receptor. J Cell Biol. 1989 Dec;109(6 Pt 1):3157–3167. doi: 10.1083/jcb.109.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczekan M. M., Juliano R. L. Internalization of the fibronectin receptor is a constitutive process. J Cell Physiol. 1990 Mar;142(3):574–580. doi: 10.1002/jcp.1041420317. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Kupfer A. The directed migration of eukaryotic cells. Annu Rev Cell Biol. 1986;2:337–365. doi: 10.1146/annurev.cb.02.110186.002005. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tapley P., Horwitz A., Buck C., Duggan K., Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989 Mar;4(3):325–333. [PubMed] [Google Scholar]
- Theret D. P., Levesque M. J., Sato M., Nerem R. M., Wheeler L. T. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J Biomech Eng. 1988 Aug;110(3):190–199. doi: 10.1115/1.3108430. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Edwards B. F., Erickson C. A. Tension in the culture dish: microfilament organization and migratory behavior of quail neural crest cells. Cell Motil. 1985;5(3):225–237. doi: 10.1002/cm.970050305. [DOI] [PubMed] [Google Scholar]
- Zahalak G. I., McConnaughey W. B., Elson E. L. Determination of cellular mechanical properties by cell poking, with an application to leukocytes. J Biomech Eng. 1990 Aug;112(3):283–294. doi: 10.1115/1.2891186. [DOI] [PubMed] [Google Scholar]
- Zaner K. S., Valberg P. A. Viscoelasticity of F-actin measured with magnetic microparticles. J Cell Biol. 1989 Nov;109(5):2233–2243. doi: 10.1083/jcb.109.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Skalak R. A continuum model of protrusion of pseudopod in leukocytes. Biophys J. 1988 Dec;54(6):1115–1137. doi: 10.1016/S0006-3495(88)83047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]