Abstract
The binding of calcium to headgroup deuterated 1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphoserine (POPS) was investigated by using deuterium magnetic resonance in pure POPS membranes and in mixed 1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphocholine (POPC)/POPS 5:1 (m:m) bilayers. Addition of CaCl2 to pure POPS bilayers led to two component spectra attributed, respectively, to liquid-crystallin POPS (less than 15 kHz) and POPS molecules in the calcium-induced dehydrated phase (cochleate) (approximately 120 kHz). The liquid-crystalline component has nearly disappeared at a Ca2+ to POPS ratio of 0.5, indicating that, under such conditions, most of the POPS molecules are in the precipitated cochleate phase. After dilution of the POPS molecules in zwitterionic POPC membranes (POPC/POPS 5:1 m:m), single component spectra characteristic of POPS in the liquid-crystalline state were observed in the presence of Molar concentrations of calcium ions (Ca2+ to POPS ratio greater than 50), showing that the amount of dehydrated cochleate PS-Ca2+ phase, if any, was low (less than 5%) under such conditions. Deuterium NMR data obtained in the 15-50 degrees C temperature range with the mixed PC/PS membranes, either in the absence or the presence of Ca2+ ions, indicate that the serine headgroup undergoes a temperature-induced conformational change, independent of the presence of Ca2+. This is discussed in relation to other headgroup perturbations such as that observed upon change of the membrane surface charge density.
Full text
PDF

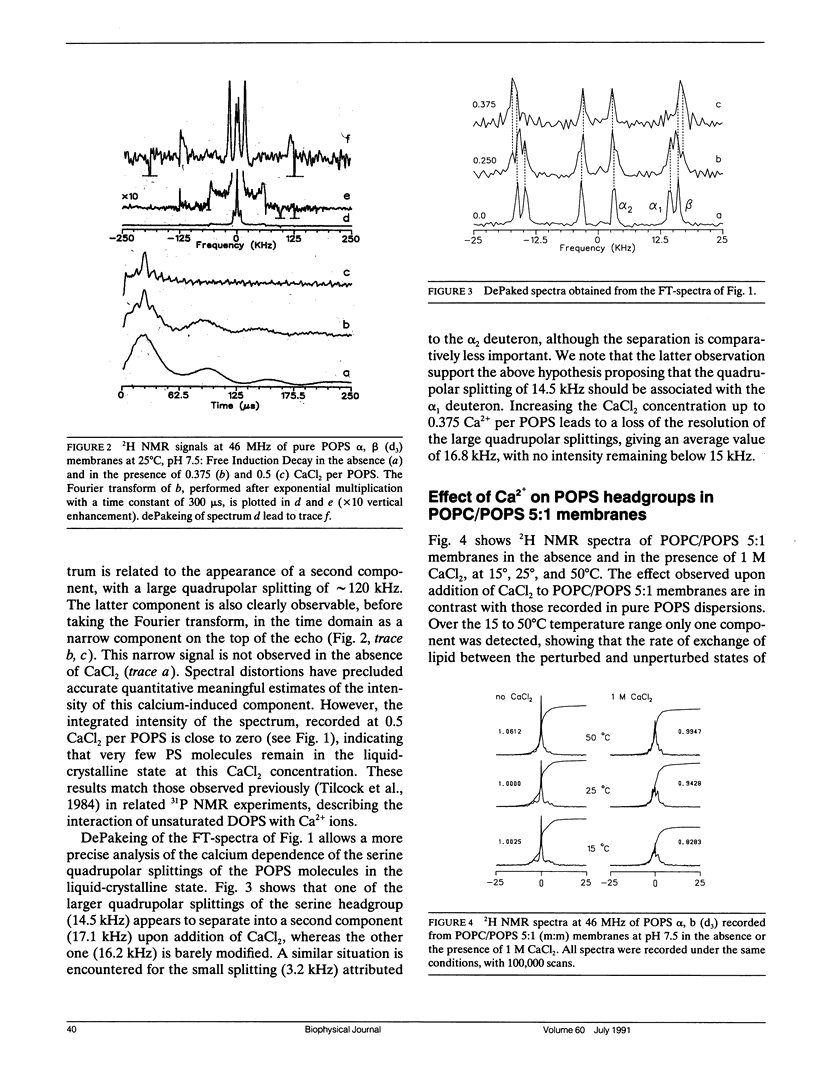
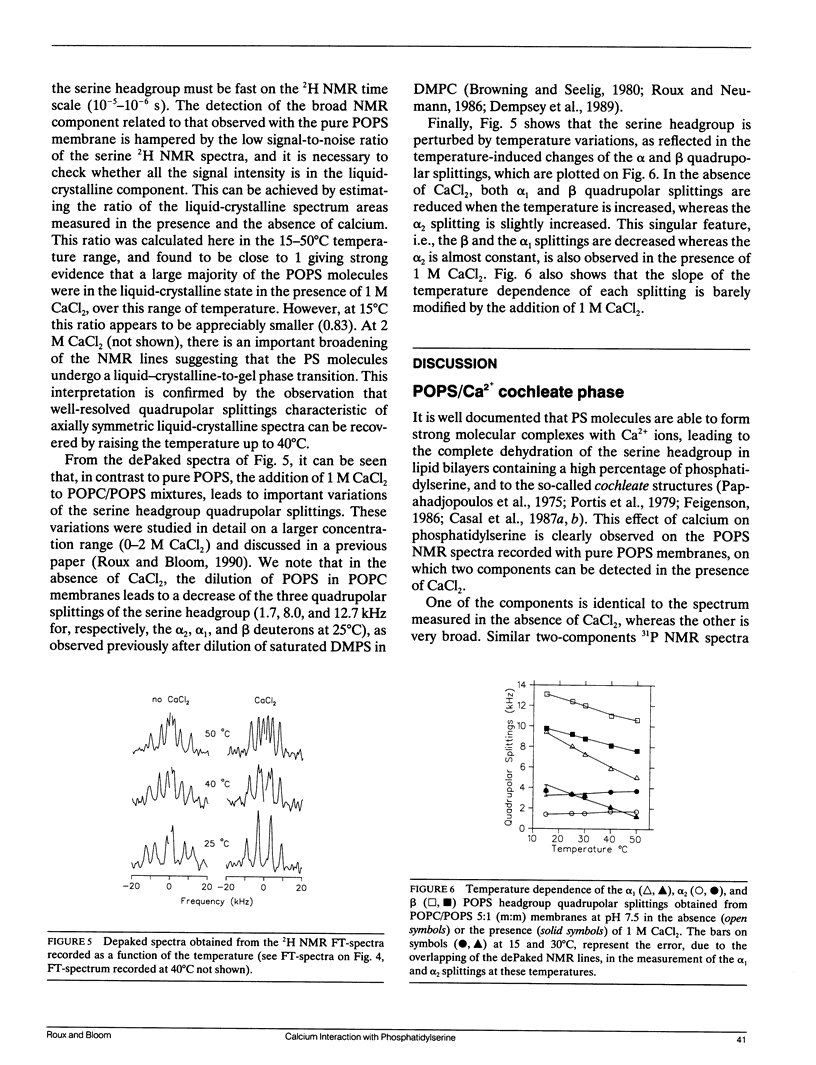



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitbol M., Dempsey C., Watts A., Devaux P. F. Weak interaction of spectrin with phosphatidylcholine-phosphatidylserine multilayers: a 2H and 31P NMR study. FEBS Lett. 1989 Feb 13;244(1):217–222. doi: 10.1016/0014-5793(89)81196-2. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Seelig J. Influence of cholesterol on the polar region of phosphatidylcholine and phosphatidylethanolamine bilayers. Biochemistry. 1978 Jan 24;17(2):381–384. doi: 10.1021/bi00595a029. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Seelig J. Bilayers of phosphatidylserine: a deuterium and phosphorus nuclear magnetic resonance study. Biochemistry. 1980 Mar 18;19(6):1262–1270. doi: 10.1021/bi00547a034. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Büldt G., Wohlgemuth R. The headgroup conformation of phospholipids in membranes. J Membr Biol. 1981 Feb 15;58(2):81–100. doi: 10.1007/BF01870972. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H., Paltauf F., Hauser H. Infrared and 31P-NMR studies of the effect of Li+ and Ca2+ on phosphatidylserines. Biochim Biophys Acta. 1987 Jun 23;919(3):275–286. doi: 10.1016/0005-2760(87)90267-0. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Martin A., Mantsch H. H., Paltauf F., Hauser H. Infrared studies of fully hydrated unsaturated phosphatidylserine bilayers. Effect of Li+ and Ca2+. Biochemistry. 1987 Nov 17;26(23):7395–7401. doi: 10.1021/bi00397a030. [DOI] [PubMed] [Google Scholar]
- Davis J. H. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys J. 1979 Sep;27(3):339–358. doi: 10.1016/S0006-3495(79)85222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Devaux P. F., Hoatson G. L., Favre E., Fellmann P., Farren B., MacKay A. L., Bloom M. Interaction of cytochrome c with mixed dimyristoylphosphatidylcholine-dimyristoylphosphatidylserine bilayers: a deuterium nuclear magnetic resonance study. Biochemistry. 1986 Jul 1;25(13):3804–3812. doi: 10.1021/bi00361a011. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. Calcium ion binding between lipid bilayers: the four-component system of phosphatidylserine, phosphatidylcholine, calcium chloride, and water. Biochemistry. 1989 Feb 7;28(3):1270–1278. doi: 10.1021/bi00429a048. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W. On the nature of calcium ion binding between phosphatidylserine lamellae. Biochemistry. 1986 Sep 23;25(19):5819–5825. doi: 10.1021/bi00367a071. [DOI] [PubMed] [Google Scholar]
- Hauser H., Shipley G. G. Interactions of divalent cations with phosphatidylserine bilayer membranes. Biochemistry. 1984 Jan 3;23(1):34–41. doi: 10.1021/bi00296a006. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. Effects of divalent cations and pH on phosphatidylserine model membranes: a 31P NMR study. Biochem Biophys Res Commun. 1980 Feb 12;92(3):846–852. doi: 10.1016/0006-291x(80)90780-9. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Mattai J., Hauser H., Demel R. A., Shipley G. G. Interactions of metal ions with phosphatidylserine bilayer membranes: effect of hydrocarbon chain unsaturation. Biochemistry. 1989 Mar 7;28(5):2322–2330. doi: 10.1021/bi00431a051. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Rosing J., Speijer H., Zwaal R. F. Prothrombin activation on phospholipid membranes with positive electrostatic potential. Biochemistry. 1988 Jan 12;27(1):8–11. doi: 10.1021/bi00401a002. [DOI] [PubMed] [Google Scholar]
- Roux M., Bloom M. Ca2+, Mg2+, Li+, Na+, and K+ distributions in the headgroup region of binary membranes of phosphatidylcholine and phosphatidylserine as seen by deuterium NMR. Biochemistry. 1990 Jul 31;29(30):7077–7089. doi: 10.1021/bi00482a019. [DOI] [PubMed] [Google Scholar]
- Roux M., Neumann J. M., Bloom M., Devaux P. F. 2H and 31P NMR study of pentalysine interaction with headgroup deuterated phosphatidylcholine and phosphatidylserine. Eur Biophys J. 1988;16(5):267–273. doi: 10.1007/BF00254062. [DOI] [PubMed] [Google Scholar]
- Roux M., Neumann J. M. Deuterium NMR study of head-group deuterated phosphatidylserine in pure and binary phospholipid bilayers. Interactions with monovalent cations Na+ and Li+. FEBS Lett. 1986 Apr 7;199(1):33–38. doi: 10.1016/0014-5793(86)81218-2. [DOI] [PubMed] [Google Scholar]
- Roux M., Neumann J. M., Hodges R. S., Devaux P. F., Bloom M. Conformational changes of phospholipid headgroups induced by a cationic integral membrane peptide as seen by deuterium magnetic resonance. Biochemistry. 1989 Mar 7;28(5):2313–2321. doi: 10.1021/bi00431a050. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R., Gruner S. M. Cation-dependent segregation phenomena and phase behavior in model membrane systems containing phosphatidylserine: influence of cholesterol and acyl chain composition. Biochemistry. 1984 Jun 5;23(12):2696–2703. doi: 10.1021/bi00307a025. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Cullis P. R. The polymorphic phase behaviour of mixed phosphatidylserine-phosphatidylethanolamine model systems as detected by 31P-NMR. Biochim Biophys Acta. 1981 Feb 20;641(1):189–201. doi: 10.1016/0005-2736(81)90583-6. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth R., Waespe-Sarcevic N., Seelig J. Bilayers of phosphatidylglycerol. A deuterium and phosphorus nuclear magnetic resonance study of the head-group region. Biochemistry. 1980 Jul 8;19(14):3315–3321. doi: 10.1021/bi00555a033. [DOI] [PubMed] [Google Scholar]


