Abstract
Using an electrostatic model for the pore and membrane region in a gramicidinlike channel, the effect of dipoles located inside the membrane on the ion transport are analyzed. Calculated energy profiles for different orientations of dipoles show a predominant influence of their radial components. The results qualitatively agree with experimental measurements of conductance on different modified gramicidins and allow to understand the important role of polar side chains on ion permeation.
Full text
PDF


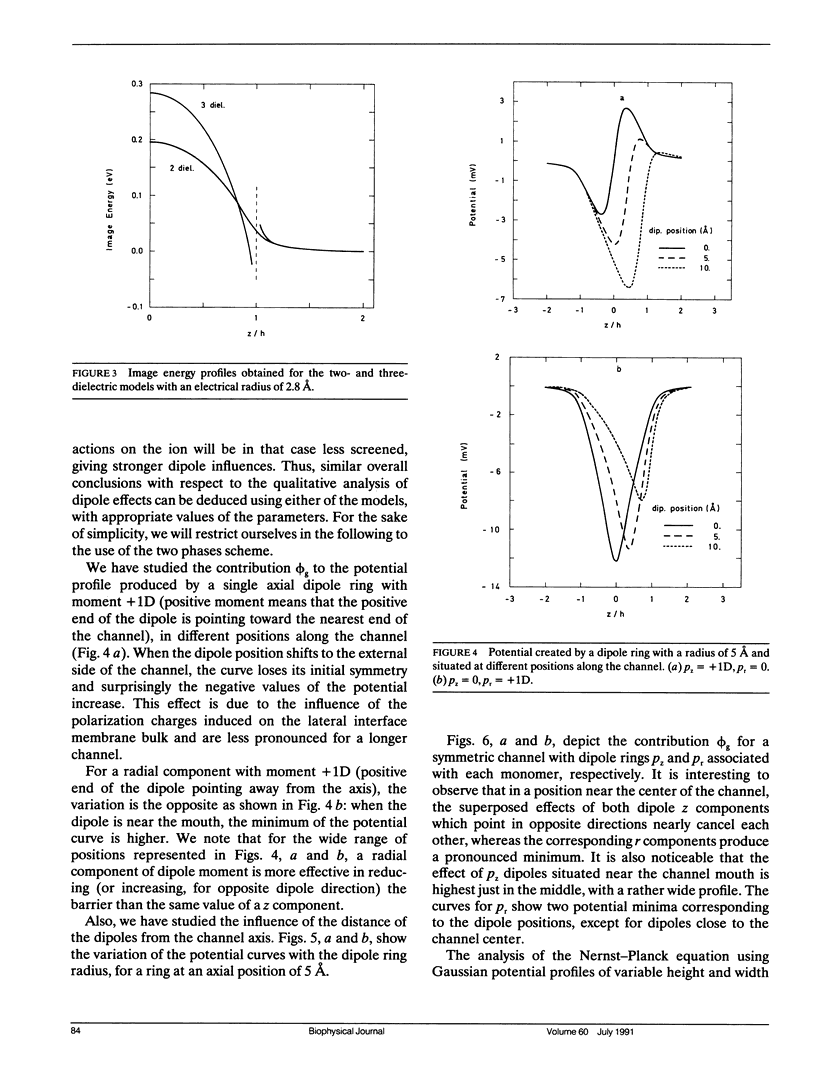
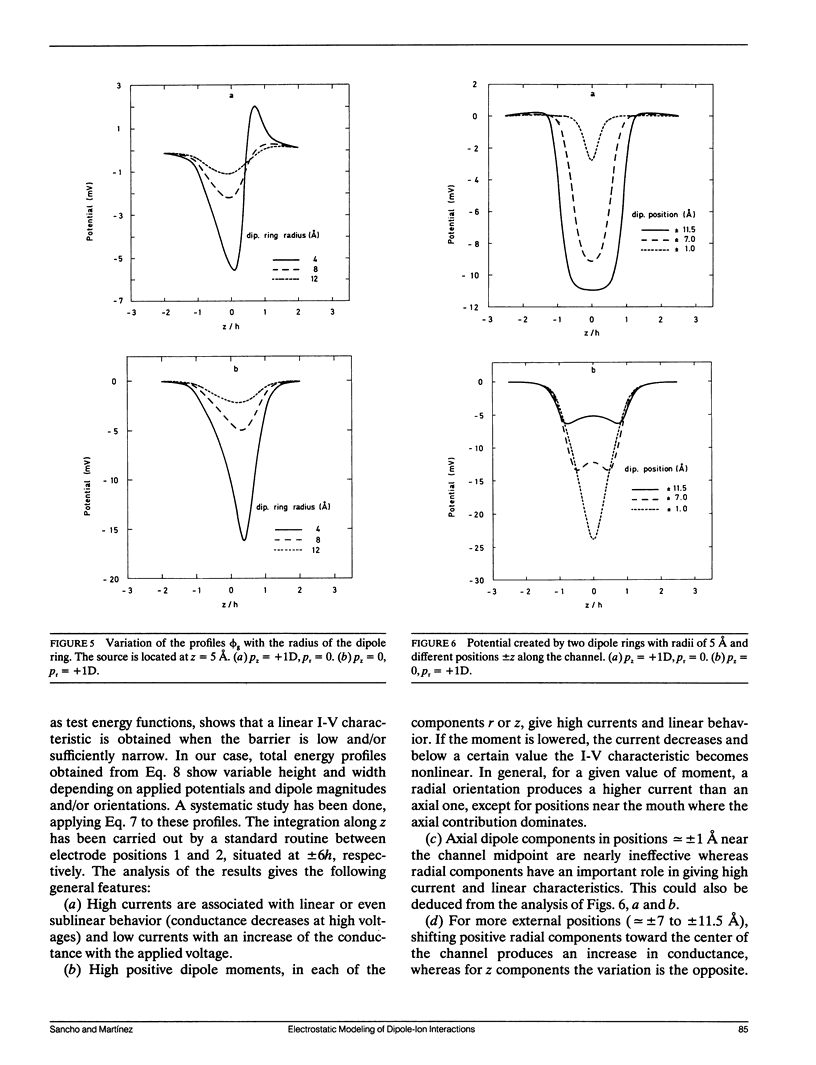



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberg E., Noda K., Gross E., Läuger P. Single-channel parameters of gramicidin A,B, and C. Biochim Biophys Acta. 1976 Jan 21;419(2):223–228. doi: 10.1016/0005-2736(76)90348-5. [DOI] [PubMed] [Google Scholar]
- Cooper K., Jakobsson E., Wolynes P. The theory of ion transport through membrane channels. Prog Biophys Mol Biol. 1985;46(1):51–96. doi: 10.1016/0079-6107(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Daumas P., Heitz F., Ranjalahy-Rasoloarijao L., Lazaro R. Gramicidin A analogs: influence of the substitution of the tryptophans by naphthylalanines. Biochimie. 1989 Jan;71(1):77–81. doi: 10.1016/0300-9084(89)90135-1. [DOI] [PubMed] [Google Scholar]
- Etchebest C., Pullman A. The effect of the amino-acid side chains on the energy profiles for ion transport in the gramicidin A channel. J Biomol Struct Dyn. 1985 Feb;2(5):859–870. doi: 10.1080/07391102.1985.10507605. [DOI] [PubMed] [Google Scholar]
- Heitz F., Spach G., Trudelle Y. Single channels of 9, 11, 13, 15-destryptophyl-phenylalanyl-gramicidin A. Biophys J. 1982 Oct;40(1):87–89. doi: 10.1016/S0006-3495(82)84462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C., Bacquet R. J., McCammon J. A., Tran P. How electrolyte shielding influences the electrical potential in transmembrane ion channels. Biophys J. 1989 Jun;55(6):1041–1052. doi: 10.1016/S0006-3495(89)82903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. Energy barriers and electric field profiles. Biophys J. 1982 Aug;39(2):157–164. doi: 10.1016/S0006-3495(82)84503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. I. Energy and potential profiles and interactions between ions. Biophys J. 1978 May;22(2):209–219. doi: 10.1016/S0006-3495(78)85485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Interpretation of biological ion channel flux data--reaction-rate versus continuum theory. Annu Rev Biophys Biophys Chem. 1986;15:29–57. doi: 10.1146/annurev.bb.15.060186.000333. [DOI] [PubMed] [Google Scholar]
- Läuger P. Microscopic calculation of ion-transport rates in membrane channels. Biophys Chem. 1982 May;15(2):89–100. doi: 10.1016/0301-4622(82)80021-5. [DOI] [PubMed] [Google Scholar]
- Matthew J. B. Electrostatic effects in proteins. Annu Rev Biophys Biophys Chem. 1985;14:387–417. doi: 10.1146/annurev.bb.14.060185.002131. [DOI] [PubMed] [Google Scholar]
- Mazet J. L., Andersen O. S., Koeppe R. E., 2nd Single-channel studies on linear gramicidins with altered amino acid sequences. A comparison of phenylalanine, tryptophane, and tyrosine substitutions at positions 1 and 11. Biophys J. 1984 Jan;45(1):263–276. doi: 10.1016/S0006-3495(84)84153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Veatch W. R., Stryer L. Transmembrane channel activity of gramicidin A analogs: effects of modification and deletion of the amino-terminal residue. J Mol Biol. 1979 Aug 25;132(4):733–738. doi: 10.1016/0022-2836(79)90386-3. [DOI] [PubMed] [Google Scholar]
- Russell E. W., Weiss L. B., Navetta F. I., Koeppe R. E., 2nd, Andersen O. S. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position 1 in gramicidin A. Biophys J. 1986 Mar;49(3):673–686. doi: 10.1016/S0006-3495(86)83694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Jordan P. C. Why is gramicidin valence selective? A theoretical study. Biophys J. 1987 Apr;51(4):661–672. doi: 10.1016/S0006-3495(87)83391-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]



