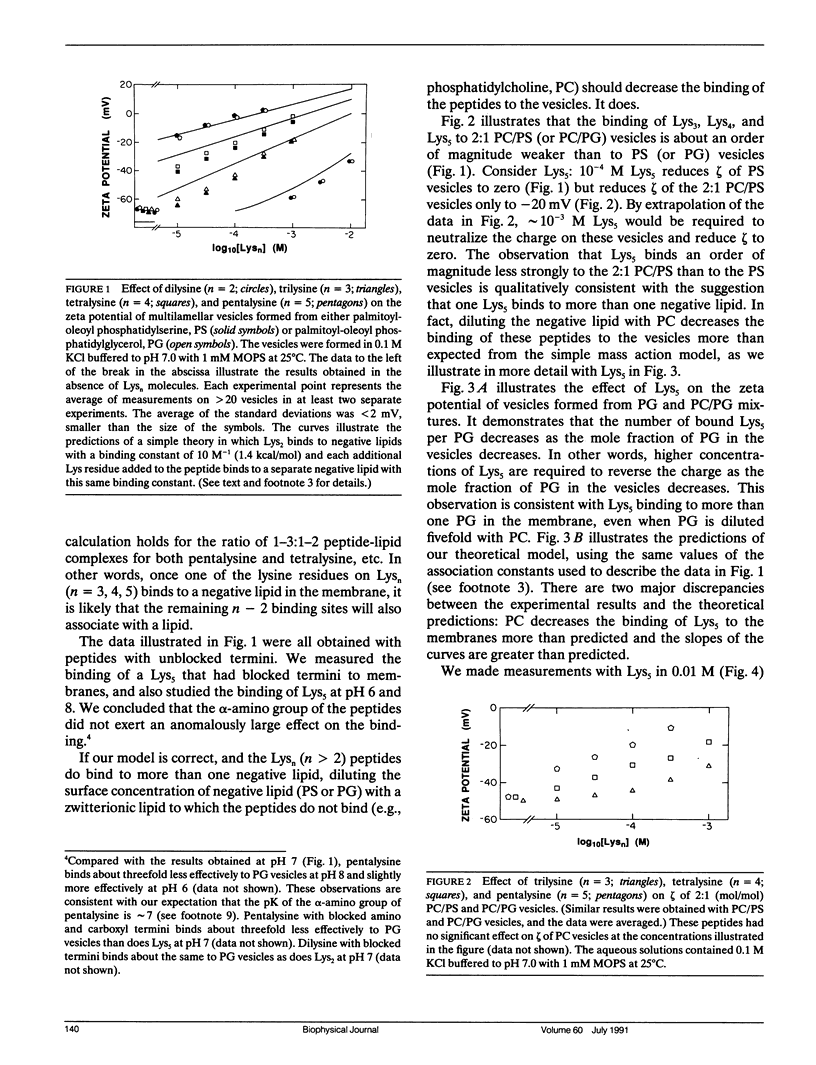
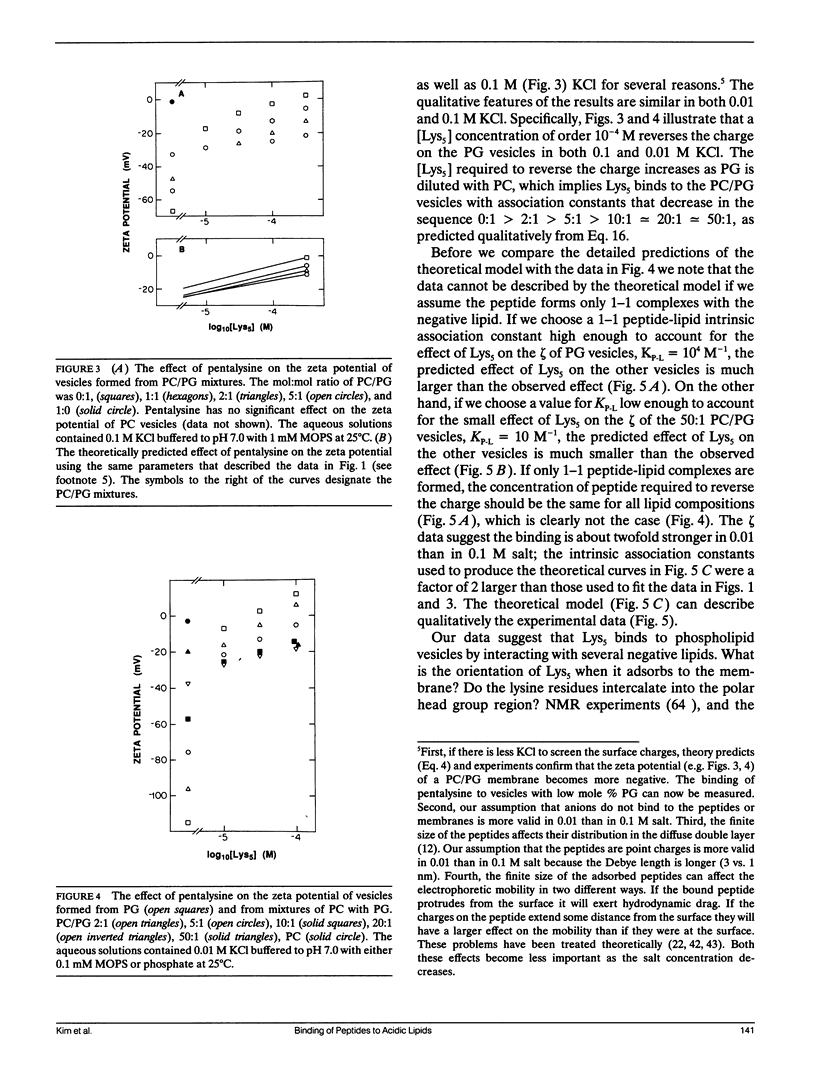
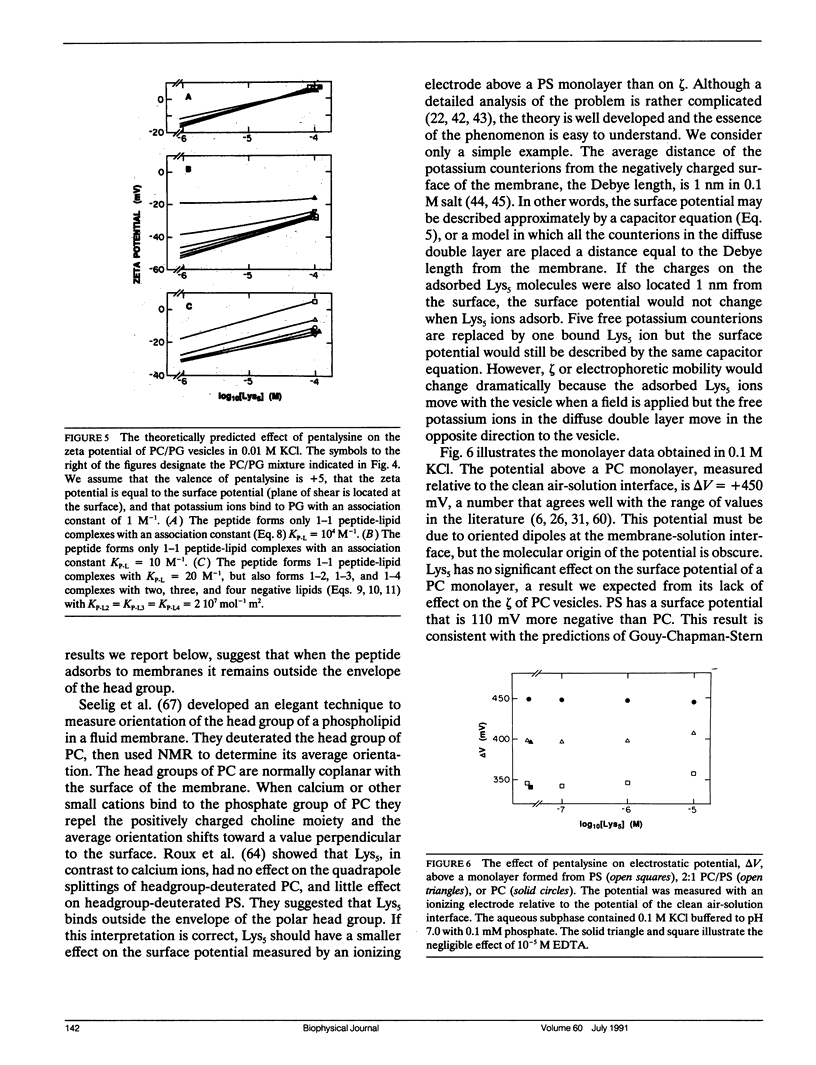
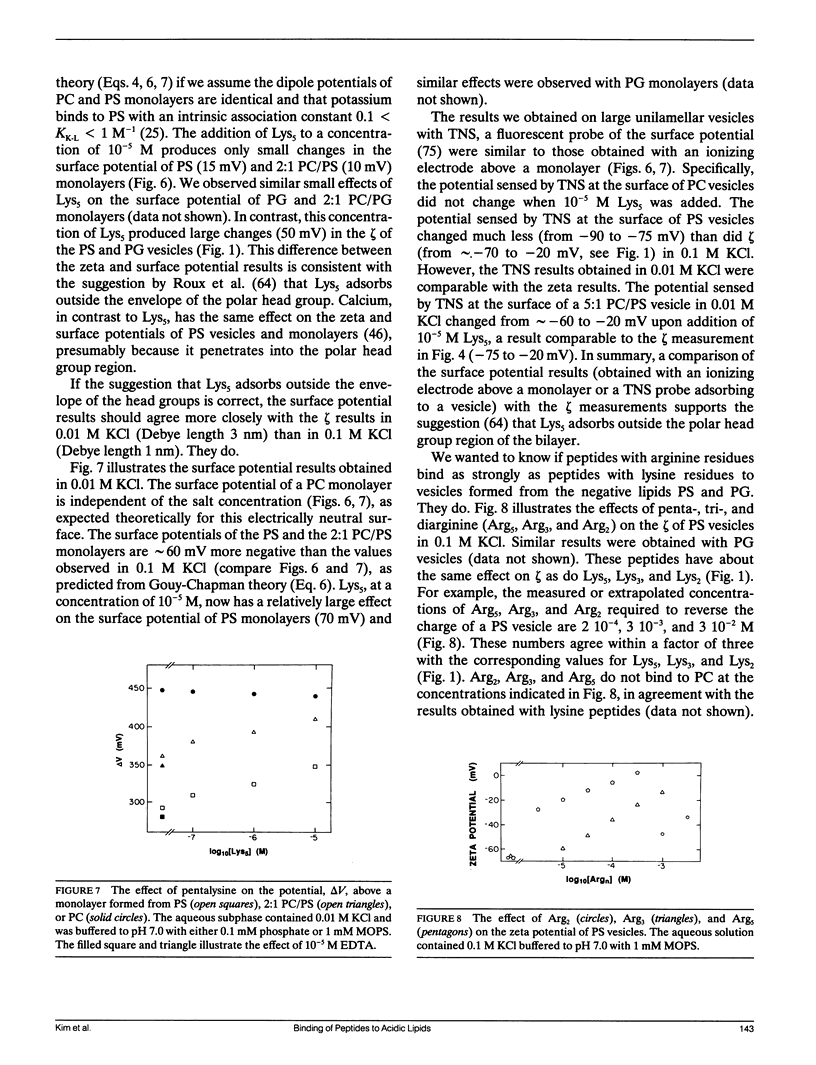
Abstract
There are clusters of basic amino acids on many cytoplasmic proteins that bind transiently to membranes (e.g., protein kinase C) as well as on the cytoplasmic domain of many intrinsic membrane proteins (e.g., glycophorin). To explore the possibility that these basic residues bind electrostatically to monovalent acidic lipids, we studied the binding of the peptides Lysn and Argn (n = 1-5) to bilayer membranes containing phosphatidylserine (PS) or phosphatidylglycerol (PG). We made electrophoretic mobility measurements using multilamellar vesicles, fluorescence and equilibrium binding measurements using large unilamellar vesicles, and surface potential measurements using monolayers. None of the peptides bound to vesicles formed from the zwitterionic lipid phosphatidylcholine (PC) but all bound to vesicles formed from PC/PS or PC/PG mixtures. None of the peptides exhibited specificity between PS and PG. Each lysine residue that was added to Lys2 decreased by one order of magnitude the concentration of peptide required to reverse the charge on the vesicle; equivalently it increased by one order of magnitude the binding affinity of the peptides for the PS vesicles. The simplest explanation is that each added lysine binds independently to a separate PS with a microscopic association constant of 10 M-1 or a free energy of approximately 1.4 kcal/mol. Similar, but not identical, results were obtained with the Argn peptides. A simple theoretical model combines the Gouy-Chapman theory (which accounts for the nonspecific electrostatic accumulation of the peptides in the aqueous diffuse double layer adjacent to the membrane) with mass action equations (which account for the binding of the peptides to greater than 1 PS). This model can account qualitatively for the dependence of binding on both the number of basic residues in the peptides and the mole fraction of PS in the membrane.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Pollard T. D. Binding of myosin I to membrane lipids. Nature. 1989 Aug 17;340(6234):565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- Alvarez O., Brodwick M., Latorre R., McLaughlin A., McLaughlin S., Szabo G. Large divalent cations and electrostatic potentials adjacent to membranes. Experimental results with hexamethonium. Biophys J. 1983 Dec;44(3):333–342. doi: 10.1016/S0006-3495(83)84307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A. D., Mason W. The effect of some general anaesthetics on the surface potential of lipid monolayers. Br J Pharmacol. 1979 Jun;66(2):259–265. doi: 10.1111/j.1476-5381.1979.tb13674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschiaschvili G., Seelig J. Melittin binding to mixed phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry. 1990 Jan 9;29(1):52–58. doi: 10.1021/bi00453a007. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Cafiso D., McLaughlin A., McLaughlin S., Winiski A. Measuring electrostatic potentials adjacent to membranes. Methods Enzymol. 1989;171:342–364. doi: 10.1016/s0076-6879(89)71019-3. [DOI] [PubMed] [Google Scholar]
- Carnie S., McLaughlin S. Large divalent cations and electrostatic potentials adjacent to membranes. A theoretical calculation. Biophys J. 1983 Dec;44(3):325–332. doi: 10.1016/S0006-3495(83)84306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier D., Pézolet M. Investigation of polylysine-dipalmitoylphosphatidylglycerol interactions in model membranes. Biochemistry. 1986 Jul 15;25(14):4167–4174. doi: 10.1021/bi00362a027. [DOI] [PubMed] [Google Scholar]
- Chung L., Kaloyanides G., McDaniel R., McLaughlin A., McLaughlin S. Interaction of gentamicin and spermine with bilayer membranes containing negatively charged phospholipids. Biochemistry. 1985 Jan 15;24(2):442–452. doi: 10.1021/bi00323a030. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Liu S. C., Lawler J., Derick L., Palek J. Identification of the protein 4.1 binding site to phosphatidylserine vesicles. Biochemistry. 1988 Jan 26;27(2):614–619. doi: 10.1021/bi00402a018. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Cohen M. Mass-action formulations of monovalent and divalent cation adsorption by phospholipid membranes. Biophys J. 1984 Oct;46(4):487–490. doi: 10.1016/S0006-3495(84)84045-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsforth G. A., Whitaker R. N., Hermans J., Lentz B. R. A new model to describe extrinsic protein binding to phospholipid membranes of varying composition: application to human coagulation proteins. Biochemistry. 1989 Sep 5;28(18):7453–7461. doi: 10.1021/bi00444a045. [DOI] [PubMed] [Google Scholar]
- Dwyer J. D., Bloomfield V. A. Binding of multivalent ligands to mobile receptors in membranes. Biopolymers. 1981 Nov;20(11):2323–2336. doi: 10.1002/bip.1981.360201104. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Gabev E., Kasianowicz J., Abbott T., McLaughlin S. Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP2). Biochim Biophys Acta. 1989 Feb 13;979(1):105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J. Binding of polylysine to charged bilayer membranes: molecular organization of a lipid.peptide complex. Biochim Biophys Acta. 1978 Jun 2;509(3):474–490. doi: 10.1016/0005-2736(78)90241-9. [DOI] [PubMed] [Google Scholar]
- Hartmann W., Galla H. J., Sackmann E. Direct evidence of charge-induced lipid domain structure in model membranes. FEBS Lett. 1977 Jun 15;78(2):169–172. doi: 10.1016/0014-5793(77)80298-6. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Elliott J. R. Surface potential changes in lipid monolayers and the 'cut-off' in anaesthetic effects of N-alkanols. Biochim Biophys Acta. 1986 Dec 16;863(2):337–340. doi: 10.1016/0005-2736(86)90278-6. [DOI] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- House C., Robinson P. J., Kemp B. E. A synthetic peptide analog of the putative substrate-binding motif activates protein kinase C. FEBS Lett. 1989 Jun 5;249(2):243–247. doi: 10.1016/0014-5793(89)80632-5. [DOI] [PubMed] [Google Scholar]
- Jones M. E., Lentz B. R. Phospholipid lateral organization in synthetic membranes as monitored by pyrene-labeled phospholipids: effects of temperature and prothrombin fragment 1 binding. Biochemistry. 1986 Feb 11;25(3):567–574. doi: 10.1021/bi00351a009. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Interactions of basic proteins with phospholipid membranes. Binding and changes in the sodium permeability of phosphatidylserine vesicles. J Biol Chem. 1971 Feb 25;246(4):1142–1148. [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Kuchinka E., Seelig J. Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry. 1989 May 16;28(10):4216–4221. doi: 10.1021/bi00436a014. [DOI] [PubMed] [Google Scholar]
- Langner M., Cafiso D., Marcelja S., McLaughlin S. Electrostatics of phosphoinositide bilayer membranes. Theoretical and experimental results. Biophys J. 1990 Feb;57(2):335–349. doi: 10.1016/S0006-3495(90)82535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche G., Carrier D., Pézolet M. Study of the effect of poly(L-lysine) on phosphatidic acid and phosphatidylcholine/phosphatidic acid bilayers by raman spectroscopy. Biochemistry. 1988 Aug 23;27(17):6220–6228. doi: 10.1021/bi00417a005. [DOI] [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- Levine S., Levine M., Sharp K. A., Brooks D. E. Theory of the electrokinetic behavior of human erythrocytes. Biophys J. 1983 May;42(2):127–135. doi: 10.1016/S0006-3495(83)84378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel R. V., Sharp K., Brooks D., McLaughlin A. C., Winiski A. P., Cafiso D., McLaughlin S. Electrokinetic and electrostatic properties of bilayers containing gangliosides GM1, GD1a, or GT1. Comparison with a nonlinear theory. Biophys J. 1986 Mar;49(3):741–752. doi: 10.1016/S0006-3495(86)83700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Mittler-Neher S., Knoll W. Phase separation in bimolecular mixed lipid membranes induced by polylysine. Biochem Biophys Res Commun. 1989 Jul 14;162(1):124–129. doi: 10.1016/0006-291x(89)91971-2. [DOI] [PubMed] [Google Scholar]
- Mosior M., McLaughlin S. Peptides that mimic the pseudosubstrate region of protein kinase C bind to acidic lipids in membranes. Biophys J. 1991 Jul;60(1):149–159. doi: 10.1016/S0006-3495(91)82038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990 Sep 21;62(6):1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and prospectives of the protein kinase c family for cellular regulation. Cancer. 1989 May 15;63(10):1892–1903. doi: 10.1002/1097-0142(19890515)63:10<1892::aid-cncr2820631005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Kour G., Marais R. M., Mitchell F., Pears C., Schaap D., Stabel S., Webster C. Protein kinase C--a family affair. Mol Cell Endocrinol. 1989 Aug;65(1-2):1–11. doi: 10.1016/0303-7207(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A. Interaction of divalent antibody with cell surface antigens. Biochemistry. 1979 Jan 23;18(2):264–269. doi: 10.1021/bi00569a004. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Radhakrishnan R., Robson R. J., Khorana H. G. The transmembrane domain of glycophorin A as studied by cross-linking using photoactivatable phospholipids. J Biol Chem. 1982 Apr 25;257(8):4152–4161. [PubMed] [Google Scholar]
- Roux M., Neumann J. M., Bloom M., Devaux P. F. 2H and 31P NMR study of pentalysine interaction with headgroup deuterated phosphatidylcholine and phosphatidylserine. Eur Biophys J. 1988;16(5):267–273. doi: 10.1007/BF00254062. [DOI] [PubMed] [Google Scholar]
- Sankaram M. B., Brophy P. J., Marsh D. Interaction of two complementary fragments of the bovine spinal cord myelin basic protein with phospholipid bilayers. An ESR spin-label study. Biochemistry. 1989 Dec 12;28(25):9692–9698. doi: 10.1021/bi00451a023. [DOI] [PubMed] [Google Scholar]
- Seelig J., Macdonald P. M., Scherer P. G. Phospholipid head groups as sensors of electric charge in membranes. Biochemistry. 1987 Dec 1;26(24):7535–7541. doi: 10.1021/bi00398a001. [DOI] [PubMed] [Google Scholar]
- Smith R., Cornell B. A., Keniry M. A., Separovic F. 31P nuclear magnetic resonance studies of the association of basic proteins with multilayers of diacyl phosphatidylserine. Biochim Biophys Acta. 1983 Aug 10;732(3):492–498. doi: 10.1016/0005-2736(83)90225-0. [DOI] [PubMed] [Google Scholar]
- Stankowski S., Schwarz G. Electrostatics of a peptide at a membrane/water interface. The pH dependence of melittin association with lipid vesicles. Biochim Biophys Acta. 1990 Jun 27;1025(2):164–172. doi: 10.1016/0005-2736(90)90094-5. [DOI] [PubMed] [Google Scholar]
- Toner M., Vaio G., McLaughlin A., McLaughlin S. Adsorption of cations to phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1988 Sep 20;27(19):7435–7443. doi: 10.1021/bi00419a039. [DOI] [PubMed] [Google Scholar]
- Walter A., Steer C. J., Blumenthal R. Polylysine induces pH-dependent fusion of acidic phospholipid vesicles: a model for polycation-induced fusion. Biochim Biophys Acta. 1986 Oct 9;861(2):319–330. doi: 10.1016/0005-2736(86)90434-7. [DOI] [PubMed] [Google Scholar]
- Winiski A. P., Eisenberg M., Langner M., McLaughlin S. Fluorescent probes of electrostatic potential 1 nm from the membrane surface. Biochemistry. 1988 Jan 12;27(1):386–392. doi: 10.1021/bi00401a058. [DOI] [PubMed] [Google Scholar]
- Winiski A. P., McLaughlin A. C., McDaniel R. V., Eisenberg M., McLaughlin S. An experimental test of the discreteness-of-charge effect in positive and negative lipid bilayers. Biochemistry. 1986 Dec 16;25(25):8206–8214. doi: 10.1021/bi00373a013. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Rietveld A., Telders N., Vaandrager B. Molecular aspects of the bilayer stabilization induced by poly(L-lysines) of varying size in cardiolipin liposomes. Biochim Biophys Acta. 1985 Nov 7;820(2):295–304. doi: 10.1016/0005-2736(85)90124-5. [DOI] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]



