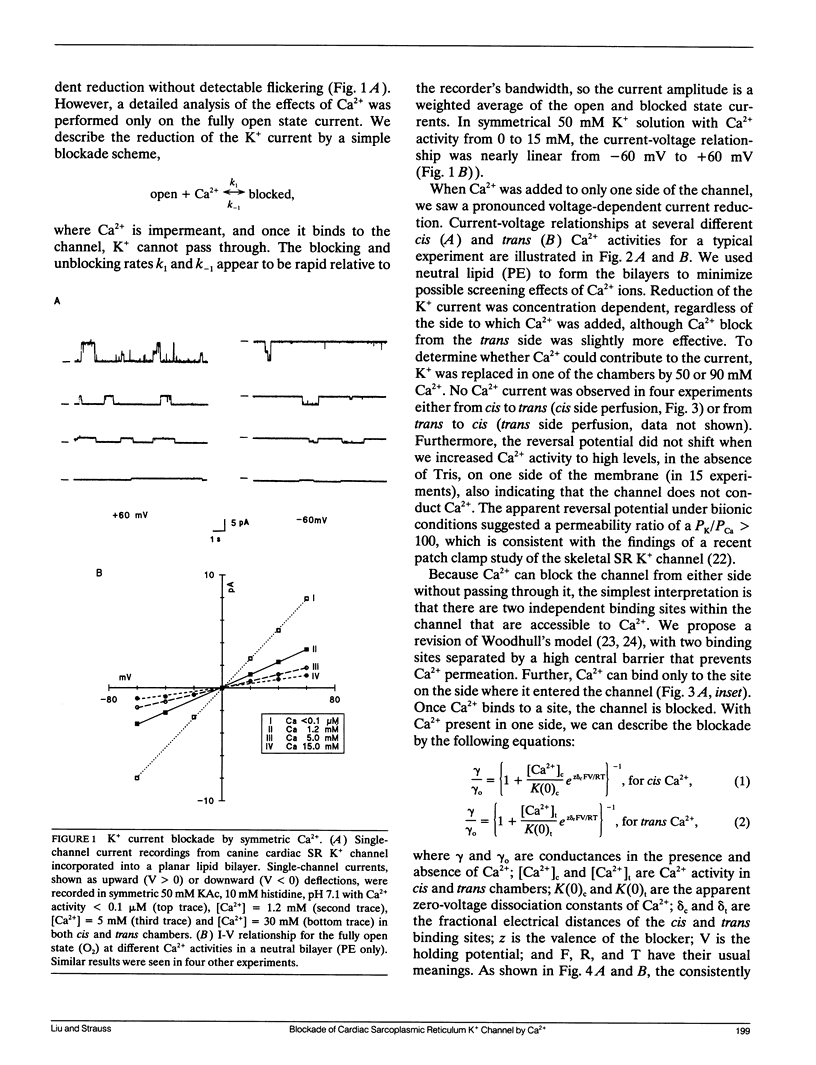
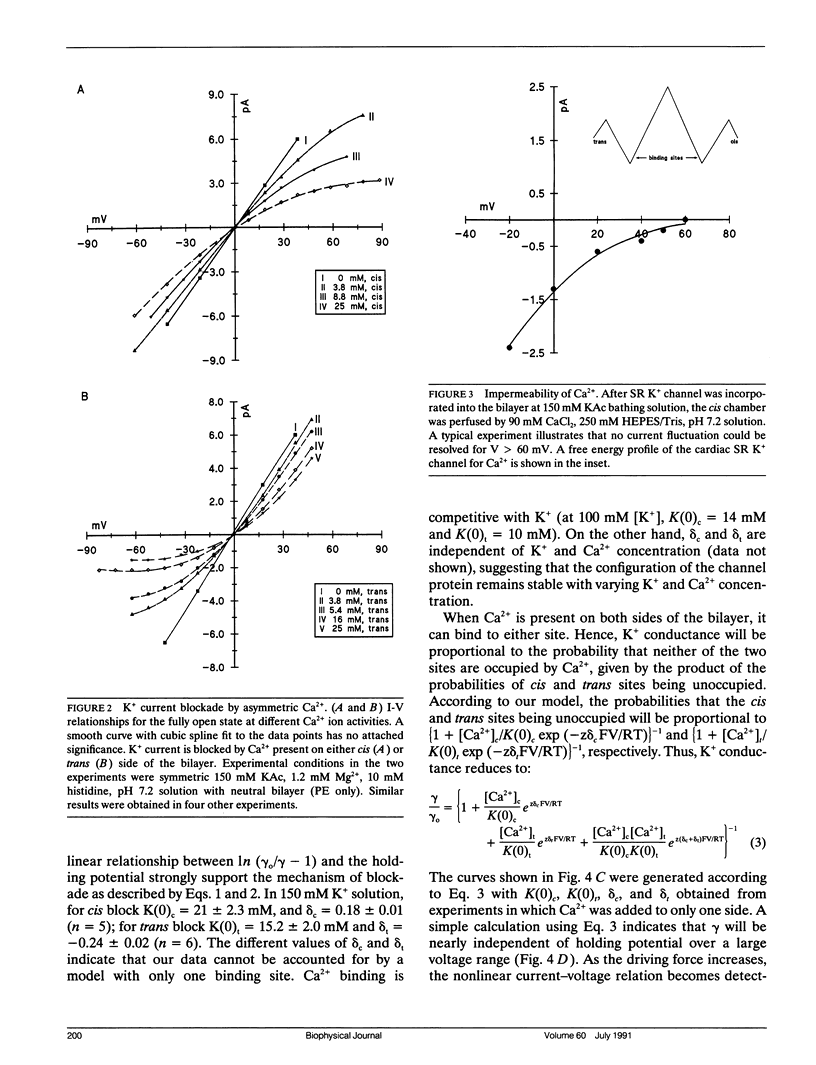
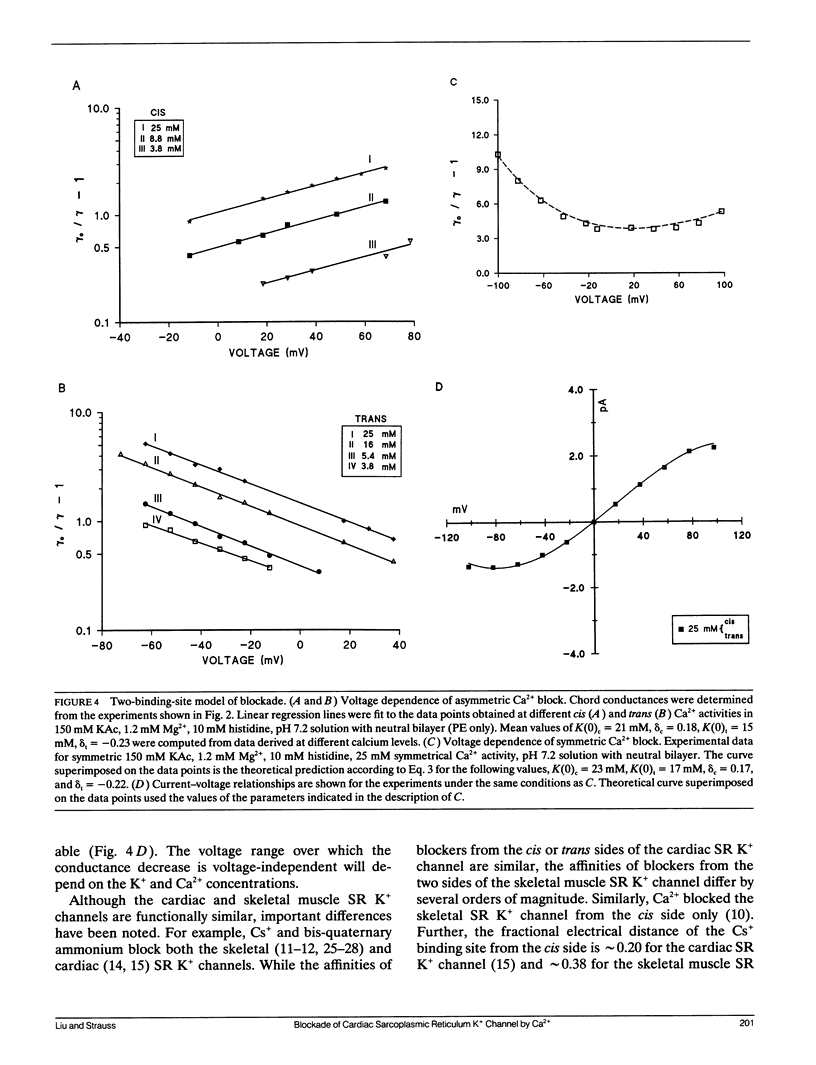
Abstract
Potassium countercurrent through the SR K+ channel plays an important role in Ca2+ release from the SR. To see if Ca2+ regulates the channel, we incorporated canine cardiac SR K+ channel into lipid bilayers. Calcium ions present in either the SR lumenal (trans) or cytoplasmic (cis) side blocked the cardiac SR K+ channel in a voltage-dependent manner. When Ca2+ was present on both sides, however, the block appeared to be voltage independent. A two-binding site model of blockade by an impermeant divalent cation (Ca2+) can explain this apparent contradiction. Estimates of SR Ca2+ concentration suggest that under physiological conditions the cardiac SR K+ channel is partially blocked by Ca2+ ions present in the lumen of the SR. The reduction in lumenal [Ca2+] during Ca2+ release could increase K+ conductance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck C. W., Best P. M. Physiological role and selectivity of the in situ potassium channel of the sarcoplasmic reticulum in skinned frog skeletal muscle fibers. J Gen Physiol. 1989 Jan;93(1):1–21. doi: 10.1085/jgp.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Calcium release and sarcoplasmic reticulum membrane potential in frog skeletal muscle fibres. J Physiol. 1984 Mar;348:209–238. doi: 10.1113/jphysiol.1984.sp015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Miller C. Decamethonium and hexamethonium block K+ channels of sarcoplasmic reticulum. Nature. 1980 Dec 4;288(5790):495–497. doi: 10.1038/288495a0. [DOI] [PubMed] [Google Scholar]
- Coronado R., Rosenberg R. L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980 Oct;76(4):425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Yellen G., Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophys J. 1985 Sep;48(3):477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Hasselbach W., Oetliker H. Energetics and electrogenicity of the sarcoplasmic reticulum calcium pump. Annu Rev Physiol. 1983;45:325–339. doi: 10.1146/annurev.ph.45.030183.001545. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Jr, Coronado R., Strauss H. C. Open-channel subconductance state of K+ channel from cardiac sarcoplasmic reticulum. Am J Physiol. 1990 Jan;258(1 Pt 2):H159–H164. doi: 10.1152/ajpheart.1990.258.1.H159. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Jr, Coronado R., Strauss H. C. Potassium channel of cardiac sarcoplasmic reticulum is a multi-ion channel. Biophys J. 1989 Jan;55(1):35–45. doi: 10.1016/S0006-3495(89)82778-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Coronado R., Miller C. Thermodynamic and kinetic studies of the gating behavior of a K+-selective channel from the sarcoplasmic reticulum membrane. J Gen Physiol. 1980 Oct;76(4):397–324. doi: 10.1085/jgp.76.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem. 1984 Feb 25;259(4):2365–2374. [PubMed] [Google Scholar]
- Meissner G., McKinley D. Permeability of canine cardiac sarcoplasmic reticulum vesicles to K+, Na+, H+, and Cl-. J Biol Chem. 1982 Jul 10;257(13):7704–7711. [PubMed] [Google Scholar]
- Meissner G. Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol Cell Biochem. 1983;55(1):65–82. doi: 10.1007/BF00229243. [DOI] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Rosenberg R. L. A voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum. Effects of transition metal ions. Biochemistry. 1979 Apr 3;18(7):1138–1145. doi: 10.1021/bi00574a003. [DOI] [PubMed] [Google Scholar]
- Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol. 1978 Apr 20;40(1):1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Oosawa Y., Sokabe M. Voltage-dependent aminoglycoside blockade of the sarcoplasmic reticulum K+ channel. Am J Physiol. 1986 Mar;250(3 Pt 1):C361–C364. doi: 10.1152/ajpcell.1986.250.3.C361. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tang J. M., Wang J., Eisenberg R. S. K+-selective channel from sarcoplasmic reticulum of split lobster muscle fibers. J Gen Physiol. 1989 Aug;94(2):261–278. doi: 10.1085/jgp.94.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J., Montgomery R. A. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80(2):191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


