Abstract
The VS ribozyme comprises five helical segments (II–VI) in a formal H shape, organized by two three-way junctions. It interacts with its stem–loop substrate (I) by tertiary interactions. We have determined the global shape of the 3–4–5 junction (relating helices III–V) by electrophoresis and FRET. Estimation of the dihedral angle between helices II and V electrophoretically has allowed us to build a model for the global structure of the complete ribozyme. We propose that the substrate is docked into a cleft between helices II and VI, with its loop making a tertiary interaction with that of helix V. This is consistent with the dependence of activity on the length of helix III. The scissile phosphate is well placed to interact with the probable active site of the ribozyme, the loop containing A730.
Keywords: FRET/metal ions/RNA catalysis/RNA folding
Introduction
RNA catalysis is important for cellular function. A number of RNA processing events, including tRNA maturation (Guerrier-Takada et al., 1983), are catalysed by ribozymes. In addition, protein synthesis appears to be catalysed by the 23S rRNA of the large ribosomal subunit (Nissen et al., 2000), and it is very likely that mRNA splicing will turn out to be RNA catalysed (Gordon et al., 2000; Yean et al., 2000). However, the fundamental chemical mechanisms of these processes are still poorly understood.
The small nucleolytic ribozymes have been valuable in the analysis of basic mechanisms of RNA catalysis, owing to their small size and simplicity. These fall into four groups, the hammerhead (Epstein and Gall, 1987; Forster and Symons, 1987), hairpin (Buzayan et al., 1986), hepatitis delta virus (HDV) (Sharmeen et al., 1988) and Varkud satellite (VS) (Saville and Collins, 1990) ribozymes. While each has a distinct structure, they all carry out essentially the same reaction, i.e. the cleavage of a specific phosphodiester linkage by a transesterification reaction involving attack of the neighbouring 2′-oxygen with departure of the 5′-oxygen. The reaction is accelerated by a factor of 105–106 by the ribozymes (Hertel et al., 1997). Where analysed, it has been found that the chirality of the phosphorus becomes inverted in the course of the reaction (van Tol et al., 1990; Koizumi and Ohtsuka, 1991), suggesting an SN2 mechanism. The reaction might be subject to a number of different catalytic strategies, including deprotonation of the 2′-hydroxyl group, facilitation of the trajectory into the in-line transition state, charge stabilization in the transition state and stabilization and/or protonation of the oxyanion leaving group. Nucleobases could act in general acid–base catalysis, exemplified by a cytosine in the HDV ribozyme (Perrotta et al., 1999; Nakano et al., 2000), while metal ions could act in general acid–base and/or electrophilic catalysis. Any such process, however, requires the correct folding of the RNA and association with the substrate to create the correct local environment in which the catalysis can proceed.
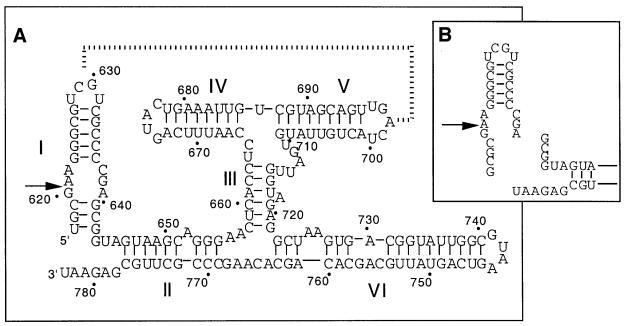
The VS ribozyme is the largest of the known nucleolytic ribozymes. It is found in the RNA transcribed from the Varkud satellite DNA of Neurospora mitochondria (Kennell et al., 1995). Cleavage occurs within the substrate stem–loop (stem I) which can be unlinked from the remaining ribozyme that consists of five helical sections (II–VI) (Figure 1). In the trans-acting form of the ribozyme, the association with the substrate occurs largely through tertiary interactions, including a long-range loop–loop interaction between helices I and V (Rastogi et al., 1996). It has also been suggested that the secondary structure of the substrate is altered upon binding to the ribozyme (Andersen and Collins, 2000). In addition to the cleavage reaction, the ribozyme can also catalyse the reverse, ligation reaction (Saville and Collins, 1991).
Fig. 1. The VS ribozyme. (A) The sequence and deduced secondary structure of the VS ribozyme (Beattie et al., 1995), drawn in its cis-acting form. The position of cleavage is indicated by the arrow. The broken line indicates a proposed tertiary interaction between the loops of helices I and V (Rastogi et al., 1996). (B) Conversion into a trans-acting ribozyme and substrate.
Crystal structures have been obtained for the hammerhead (Pley et al., 1994; Scott et al., 1995), hairpin (Rupert and Ferré-D’Amaré, 2001) and HDV (Ferré-D’Amaré et al., 1998) ribozymes, and these have been extremely useful in refining ideas concerning catalytic mechanisms. However, there is at present relatively little structural information on the VS ribozyme. The secondary structure of the ribozyme section (Beattie et al., 1995) shows that the five helical sections are organized by two three-way junctions as a formally H-shaped structure (Figure 1). Experience with a number of RNA species indicates that helical junctions can play an important role as structural features that organize the conformation on a large scale. For example, the hairpin ribozyme requires the association of two loops for activity; these are carried on two adjacent arms of a perfect four-way junction, the presence of which greatly enhances the efficiency of folding into the active form of the ribozyme (Murchie et al., 1998; Walter et al., 1999; Zhao et al., 2000).
We have therefore embarked on an examination of the structure of the two three-way junctions of the VS ribozyme, with the goal of defining the global fold of the complete five-helix ribozyme. In a previous paper, we presented the structure of the lower (2–3–6) junction (Lafontaine et al., 2001a) based on comparative gel electrophoresis, long-range distance information derived from FRET, and homology-based modelling. We found that this junction undergoes metal ion-induced folding into a structure in which helices III and VI are co-axially stacked, and a small angle is subtended between helices II and VI. In the present work, we have determined the global shape of the 3–4–5 junction of the ribozyme when folded in Mg2+ ions, and the dihedral angle between the two junctions. This defines the general fold of the ribozyme, and allows us to deduce the location of the substrate stem–loop. We propose that the substrate stem–loop is docked into the cleft between helices II and VI, where it interacts with the probable active site of the ribozyme, the A730 loop (Lafontaine et al., 2001b).
Results and discussion
The global structure of the 3–4–5 junction
The overall shape of the VS ribozyme, comprising helices II–VI, will be largely determined by the conformation of the two three-way helical junctions. We have previously determined the global shape of the 2–3–6 junction, and we have therefore now turned to the remaining 3–4–5 junction that relates helices III, IV and V. This is an HS1HS5HS2 junction (Lilley et al., 1995), i.e. it contains one or more formally unpaired nucleotides linking each of the pairs of helices around the centre. Each of the helices comprises three or more uninterrupted Watson–Crick base pairs from the centre of the junction. We have analysed the trajectories of the arms around the junction using comparative gel electrophoresis and FRET (Lilley, 2000).
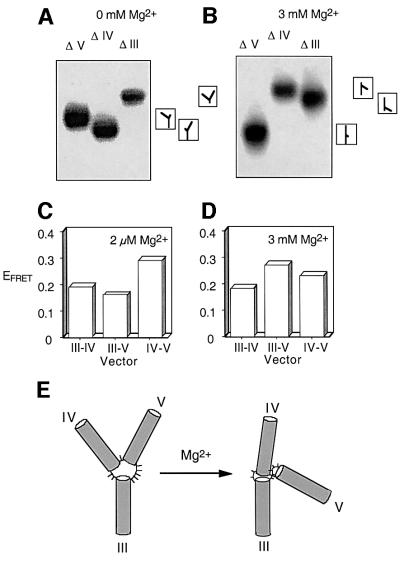
Analysis of the structure of a three-way junction by comparative gel electrophoresis requires the derivation of the three species comprising two long (extended by randomly chosen sequences to a total length of 40 bp) and one short (11 bp) helical arm, and comparison of their electrophoretic mobilities in a polyacrylamide gel under non-denaturing conditions. The relative mobilities can be related directly to the size of the angle subtended between the two long arms. The component oligonucleotides were generated by transcription, and thus the molecules were entirely composed of RNA. All three helices were perfectly base paired, and thus the bulged adenine of helix III was removed. These are named from the shortened arm; e.g. ΔIII has long arms IV and V, and a short arm III. Electrophoresis was performed in the presence of 0 and 3 mM Mg2+ ions (Figure 2A and B). It can be seen that, in common with most such branched nucleic acids, the structure changes on addition of divalent metal ions. In the absence of added ions, the mobilities increase in the order ΔIII<ΔV<ΔIV, indicating that the angles between helices increase in magnitude in the order IV–V<III–IV<III–V. This corresponds to the size of the unpaired stretch of nucleotides at the three points, and is therefore consistent with an extended structure with no interhelical stacking of arms. This is typical for three-way junctions in both RNA (Bassi et al., 1995, 1997; Lafontaine et al., 2001a) and DNA (Welch et al., 1993). Upon addition of Mg2+ ions, the pattern of mobilities is consistent with a global structure in which the largest angle is subtended between helices III and IV, and the smallest angle lies between helices III and V.
Fig. 2. The global structure of the 3–4–5 junction. (A and B) Comparative gel electrophoresis. The three possible species having two long arms of 40 bp and one shorter arm of 11 bp were created from transcribed RNA, and named by the shortened arm (e.g. species ΔIII has arm III of 11 bp). The three species were electrophoresed in a polyacrylamide gel in the presence of the indicated metal ion concentrations. The junctions were radioactively 5′-32P-labelled, and the different species were revealed by phosphorimaging of dried gels. The experiment was repeated in the presence of 0 (A) and 3 mM (B) Mg2+ ions. The differences in the patterns of mobility clearly indicate an ion-induced structural transition that alters the global structure of the junction. The interpretations of the mobility patterns are shown to the right of the phosphorimages, corresponding to the global structures illustrated in (E) (see text). (C and D) FRET analysis. We have constructed three species with three arms of 11 bp, each carrying donor (fluorescein) and acceptor (Cy3) fluorophores attached to the 5′ termini of two helical arms. The species are named according to the helical arms carrying the donor and acceptor, in that order. Histograms of FRET efficiency (EFRET) for three end-to-end vectors, measured in the presence of 2 µM (C) or 3 mM (D) Mg2+ ions, are shown. Relative FRET efficiencies agree qualitatively with the global model illustrated in (E). (E) A model for the global folding of the VS 3–4–5 junction. The structure is extended at low divalent ion concentration. On addition of 3 mM Mg2+ ions, the structure undergoes a folding transition that involves movement of helix V, shortening the III–V end-to-end distance.
The structure of the 3–4–5 junction was also analysed using FRET. Efficiency of energy transfer (EFRET) between donor–acceptor pairs separated by distance R is given by (Förster, 1948):
EFRET = [1 + (R/R0)6]–1(1)
In this approach, we have compared the efficiency of energy transfer between fluorescein and Cy3 fluorophores (for which R0 = 56 Å; Norman et al., 2000) attached to the 5′ termini of different pairs of helical arms, each 11 bp in length (omitting A718). FRET efficiencies were measured in the presence of 2 µM and 3 mM Mg2+ ions, and the results are presented in Figure 2C and D. The differences between the two patterns of efficiencies confirm the dependence of the structure on divalent metal ions. Moreover, if a simple relationship between end-to-end distance and FRET efficiency is assumed, the results are in excellent agreement with those of the comparative gel electrophoresis.
Thus, both methods indicate that the junction adopts an extended structure in the absence of divalent metal ions, and undergoes a folding process on addition of Mg2+ ions. The large angle subtended between helices III and IV might indicate co-axial stacking of these arms; the value of EFRET = 0.18 for the III–IV vector is similar to that measured for a 22 bp duplex (Lafontaine et al., 2001a), where EFRET = 0.20.
Ion-induced folding of the 3–4–5 junction
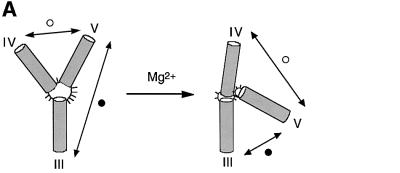
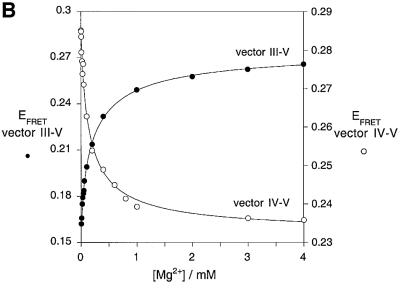
The folding of junction 3–4–5 may be followed via the change in EFRET for chosen end-to-end vectors on titration of Mg2+ ions (Figure 3). We have compared vectors III–V and IV–V, since these become shorter and longer, respectively, on folding. Similar analysis of the III–IV vector shows a small change in EFRET at a lower metal ion concentration (a reduction of 0.01 occurring at 0–10 nM), corresponding to a different aspect of the transition (data not shown). The data for vectors III–V and IV–V have been fitted to a two-state conformational transition induced by the binding of Mg2+ ions with a Hill coefficient n and an apparent association constant KA. From these, we can calculate the Mg2+ ion concentration ([Mg2+]1/2 = (1/KA)1/n) at which the transition is 50% complete. The proportion of folded junction (α) is given by:
Fig. 3. Folding of the VS junction 3–4–5 as a function of Mg2+ concentration. (A) Scheme of the proposed folding of the junction. In this experiment, we analyse the shortening of the III–V vector (filled circle) and the concomitant lengthening of the IV–V vector (open circle). (B) FRET efficiencies for the III–V (filled circles) and IV–V (open circles) vectors are plotted as a function of Mg2+ concentration. The experimental data were fitted (lines) by regression to a two-state model where binding of metal ions induces a structural change.
α = KA·[Mg2+]n/(1 + KA·[Mg2+]n)(2)
Good fits were obtained with this simple model, giving a value of n = 1.0 ± 0.1 and 0.9 ± 0.1, and [Mg2+]1/2 = 260 and 320 µM, for the vectors 3–5 and 4–5, respectively. Thus the 3–4–5 junction folds in response to the non-cooperative binding of Mg2+ ions. We found previously that the folding of the 2–3–6 junction is also induced by non-cooperative binding of Mg2+ ions (Lafontaine et al., 2001a). The [Mg2+]1/2 was a little lower for the 2–3–6 junction at ∼100 µM, but in a similar range nevertheless. Thus the folding properties of the two three-way junctions of the VS ribozyme are similar, and the complete ribozyme should therefore fold over a relatively narrow ionic concentration range.
Sequence changes in the 3–4–5 junction affect catalytic activity
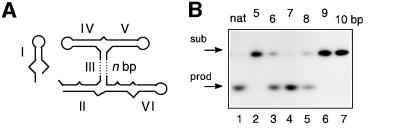
We have made substitutions in all the nucleotides that form the 3–4–5 junction, and examined the effect on the cleavage rate in the context of the complete trans-acting ribozyme (Figure 4). Cleavage rates were measured under single-turnover conditions for the ribozyme 1 plus substrate 1 combination (Lafontaine et al., 2001a), and the observed rate constants are collected in Table I. Similar studies have been carried out recently on the cis-acting form of the ribozyme (Sood and Collins, 2001).
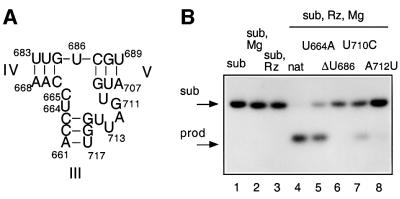
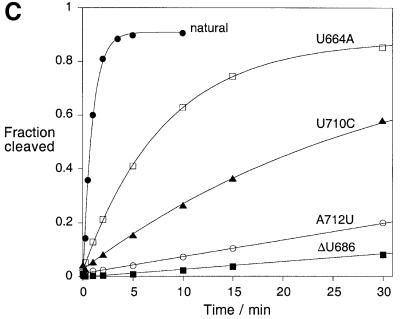
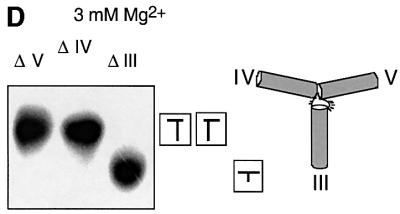
Fig. 4. Effect of sequence changes at the 3–4–5 helical junction on ribozyme cleavage activity in trans. (A) The sequence of the 3–4–5 junction. (B) Separation of substrate and product for the natural ribozyme and selected sequence variants. Radioactively 5′-32P-labelled substrate (∼1 nM) was incubated with an excess of ribozyme (1 µM) for 15 min in the presence of 10 mM Mg2+ ions. At this ribozyme concentration, the observed cleavage rates include contributions from both complex formation and catalysis. After termination, the substrate (arrowed, sub) and product (prod) were separated by gel electrophoresis and visualized by autoradiography. Tracks 1–3, control experiments; 1, unincubated substrate; 2, substrate incubated alone with Mg2+ ions; 3, substrate plus ribozyme incubated in the absence of Mg2+ ions; tracks 4–8, substrate plus ribozyme incubated in the presence of Mg2+ ions; 4, natural sequence; 5, U664A; 6, ΔU686; 7, U710C; 8, A712U. (C) Progress curves for cleavage reactions. Plots of cleaved fraction as a function of time for natural sequence (filled circles) and U664A (open squares); ΔU686 (filled squares); U710C (filled triangles); A712U (open circles) ribozymes. The time scale has been chosen to show the progress of the variant ribozymes, and thus the data for the natural ribozyme are compressed. (D) The effect of deletion of U686 on the conformation of the 3–4–5 junction. Comparative gel electrophoresis was carried out on this variant analogously to that in Figure 2. The pattern of mobilities is interpreted in terms of the global structure shown schematically on the right.
Table I. Effects of sequence changes in the 3–4–5 junction and surrounding helices on cleavage activity.
| VS ribozyme variant | kobs/min |
|---|---|
| Natural sequence | 1.0 |
| Mutations in the 3–4–5 junction | |
| U664A | 0.12 |
| C665A | 0.015 |
| U686A | 0.006 |
| ΔU686 | <0.001 |
| U710C | 0.03 |
| U710A | 0.002 |
| G711U | 0.9 |
| A712U | 0.005 |
| A712G | 0.006 |
| U713A | 0.02 |
| U714A | 0.14 |
| Proximal base pairs | |
| C663G:G715C | 0.27 |
| C666G:G685C | 0.03 |
| C687G:G709C | 0.008 |
| Stem III | |
| U659A:A720U | 0.8 |
| C660G:G719C | 1.2 |
| A661U:U717A | 0.6 |
| C662G:G716C | 0.6 |
| Stem III reversea | 0.04 |
| Stem III reverse, except A718b | 0.5 |
| A718 reversec | 0.12 |
| ΔA718d | 0.14 |
| +A after C660e | 0.77 |
| Length 5 bpf | <0.001 |
| Length 6 bpf | 0.14 |
| Length 7 bpf | 0.85 |
| Length 8 bpf | 0.09 |
| Length 9 bpf | 0.0037 |
| Length 10 bpf | <0.001 |
| Stem IV | |
| Stem IV, 6 bpg | 0.49 |
| Stem IV, 4 bph | 0.61 |
| Stem IV, 2 bpi | 0.012 |
| Stem V | |
| U705Cj | 0.9 |
| U708Cj | 0.9 |
| Stem V, 3 bpk | <0.001 |
| Stem V, 5 bpl | <0.001 |
| Stem V, 7 bpm | 0.047 |
| Stem V, 10 bpn | 0.031 |
| Stem V, 11 bpo | 0.0032 |
| Stem V, 12 bpp | <0.001 |
| Stem V, 13 bpq | <0.001 |
aEach nucleotide of stem III (including the bulged A718) relocated to the opposite strand.
bEach nucleotide of stem III except for A718 relocated to the opposite strand.
cA718 relocated to the opposite strand, i.e. inserted in between C660 and A661.
dDeletion of the bulged A in stem III; this is identical to the ribozyme with stem III of 6 bp.
eInsertion of an additional A between C660 and A661 to create an A·A mismatch.
fThe length of helix III was varied between 5 and 10 bp as indicated in Materials and methods. In each case, there is no bulged nucleotide.
gShorten helix IV to 6 bp by removal of nucleotides 671–672 and 679–680.
hShorten helix IV to 4 bp by removal of nucleotides 669–672 and 679–682.
iShorten helix IV to 2 bp by removal of nucleotides 667–672 and 679–684.
jChange of G·U mispair to G–C base pair.
kShorten helix V to 3 bp by removal of nucleotides 688–693 and 703–708.
lShorten helix V to 5 bp by removal of 688–691 and 705–708.
mShorten helix V to 7 bp by removal of nucleotides 688–689 and 707–708.
nExtend helix V to 10 bp by addition of G after C692 and C after U703.
oExtend helix V to 11 bp by addition of GU after C692 and AC after U703.
pExtend helix V to 12 bp by addition of GUC after C692 and GAC after U703.
qExtend helix V to 13 bp by addition of GUCU after C692 and AGAC after U703.
Each of the strands linking the arms of the junction have one or more formally unpaired nucleotides. The single uridine lying between helices IV and V is particularly important, since either substitution or deletion lead to large reductions in cleavage activity. The two pyrimidine nucleotides linking arms III and IV are less important, although the activity of C665U is reduced by 67-fold. The five-nucleotide stretch linking arms III and V exhibits markedly differing sensitivities. G711U has virtually full activity, and U714A is only reduced 7-fold. However, the activity of variants altered at U710, A712 or U713 is reduced by factors of 30–500. We have also examined the effect of individually reversing the junction-proximal base pairs of each of the three arms. The effects on substrate cleavage were significant, particularly for the base pair at the end of helix V (C687–G709). This is in contrast to our earlier experiments on junction 2–3–6, where reversal of the terminal base pairs had negligible effects on cleavage rates (Lafontaine et al., 2001a). The results indicate that the local structure of the 3–4–5 junction is likely to involve multiple interactions, and that nucleotides U686, U713, A712 and U710 probably participate in important interactions. Sood and Collins (2001) have recently proposed that nucleotides 710–712 form a uridine turn. In addition, the C687–G709 base pair may be disrupted, or involved in a tertiary interaction.
Deletion of the single uridine between helices IV and V alters the conformation of the 3–4–5 junction
Of all the sequence variants in and around the 3–4–5 junction, deletion of U686 had the largest effect on the catalytic rate of the ribozyme. This is the formally single-stranded nucleotide, located at the junction between helices IV and V. We therefore carried out a comparative gel electrophoretic analysis of this variant junction. The species with two long and one short arm were made exactly as before except for the deletion of this single nucleotide. The pattern of mobilities in the presence of Mg2+ ions (Figure 4D) is clearly different from that of the natural sequence (compare with Figure 2B), showing that the folded structure is significantly altered by the change in sequence. Thus there is a correlation between the effect of the removal of this base on the activity of the ribozyme and its effect on the conformation of the 3–4–5 junction. The pattern of mobilities can be interpreted in terms of a structure in which the largest angle is subtended between helices IV and V. The single uridine probably provides the conformational flexibility to allow helices III and IV to interact co-axially in the natural sequence. In its absence, helix IV is forced to interact with helix V, changing the overall geometry of the junction and thus perturbing the interaction with the substrate.
The dependence of catalytic activity on helices IV and V
We have carried out a limited exploration of the length requirements for helices IV and V in the function of the ribozyme in trans. The observed rates of the modified species under single turnover conditions are presented in Table I.
Stem IV comprises a perfect 8 bp helix, terminated by a tetraloop. It is very tolerant of deletion. Shortening to 4 bp only reduces the activity to 60% of the natural rate, and measurable activity is even retained when it is reduced to only 2 bp. We conclude that the only function of stem IV is to allow the formation of the 3–4–5 junction.
Stem V comprises 9 bp, including two G·U mismatches; however, these do not appear to be important, since individual alteration to G–C base pairs (i.e. U705C and U708C) barely reduced the activity of the ribozyme. However, in contrast to helix IV, changes in length of helix V resulted in a significant reduction in cleavage activity. Addition of 1 or 2 bp to helix V leads to a 30- and 300-fold reduction in the rate of cleavage, respectively, while removal of 2 bp led to a 20-fold lowering of cleavage rate. Larger additions or deletions led to the loss of measurable cleavage activity. Rastogi et al. (1996) have indicated that the loop of stem V interacts with that of stem I, and is therefore required for the binding of the substrate and altering its secondary structure (Andersen and Collins, 2000). The tight requirement for stem V to be of the correct length can be understood in terms of the interaction with the substrate; addition or removal of base pairs will alter the length, but, probably more importantly, result in a rotation of the loop that will significantly change the interaction with the substrate loop. Similar results have been obtained using the cis-acting form of the ribozyme (Rastogi and Collins, 1998).
The sequence of helix III is not important for catalytic activity
We have examined the requirement for the natural sequence of helix III on the function of the ribozyme in trans, and the measured rates are presented in Table I. In the natural ribozyme, this helix comprises 6 bp, with a single adenine bulge on the 3′ strand. Each of the 6 bp was reversed individually, but in no case did we observe a change in activity by a factor >2. Even reversal of all 6 bp, while retaining the adenine bulge (A718) on the 3′ strand only led to a 2-fold reduction in activity. However, when the entire sequence of helix III was reversed including the adenine bulge (thus transferring the bulge to the 5′ strand), this led to a 25-fold reduction in cleavage rate. In contrast, complementation of the adenine bulge (making a perfect 7 bp helix) had almost no effect on activity. Thus the sequence of stem III is completely unimportant for ribozyme activity; the only feature of significance is the orientation of the bulge when present. However, the length of this helix is very important, as we discuss below.
Global structure of the VS ribozyme (helices II–V)
The basic structure of the ribozyme may be constructed by bringing together the two three-way junctions through their common helix III, generating an approximately co-linear axis for helices IV, III and VI (modelled below; refer to Figure 6). We have established the global structures of both junctions, but one main uncertainty remains. The rotational directions of helices II and V with respect to helix III are not known for the junctions individually, and thus the dihedral angle between these two arms in the five-helix ribozyme is unknown. We have therefore devised an electrophoretic experiment designed to estimate this angle in the complete ribozyme. The principle of this experiment was to compare a series of species in which the longest arms were stems II and V. By varying the length of stem III, we could systematically change the dihedral angle between helices II and V. This would alter the global shape of the molecule, and thus the expected electrophoretic mobility.
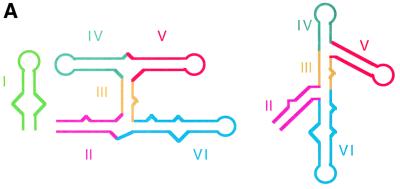
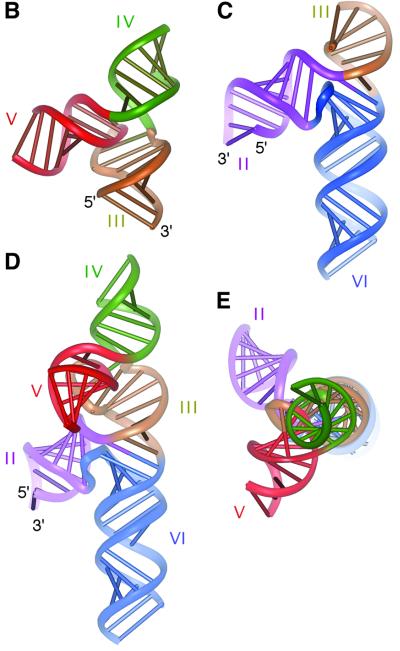
Fig. 6. Constructing the structure of the VS ribozyme by molecular modelling. (A) Schematic of the secondary structure of the VS ribozyme, with each helix differentiated by colour (used in the modelled structures here and in Figure 7). This has been drawn in the conventional manner (left), and in a way that corresponds more closely to the global structure deduced in these studies (right). (B) The structure of the 3–4–5 junction, modelled using molecular graphics guided by the global structure deduced from comparative gel electrophoresis and FRET. (C) The structure of the 2–3–6 junction. This image was made using our earlier model of this junction based on FRET-derived distances, and comparison with the 5–6–7 junction of the 23S rRNA (Lafontaine et al., 2001a). (D and E) A model for the complete five-helix ribozyme. This was derived by assembling the two three-way junctions through their common helix III, using the dihedral angle between helices II and V measured by gel electrophoresis (see text and Figure 5). Two views of the model are shown, a face view (D) and a view along the axis of helix III (E). In constructing these molecular graphics images, no specific representation of the bulges or internal loops have been made due to a lack of specific data on these structures at this time.
Using RNA molecules transcribed from DNA templates constructed by PCR, we have generated a series of ribozyme variants in which helix III was perfectly base paired, and varied in length from 6 to 20 bp (Figure 5A). Helices VI and IV were shortened to 6 bp stem–loop structures, while helices II and V were extended by random sequences, removing the loops and bulges to create perfectly base paired helices. The length of these helices was 40 bp in the species in which helix III was 6 bp, and were then both reduced by 1 bp for every 2 bp added to helix III. Thus the contour length of the complete helix II–III–V path was kept constant through the series.
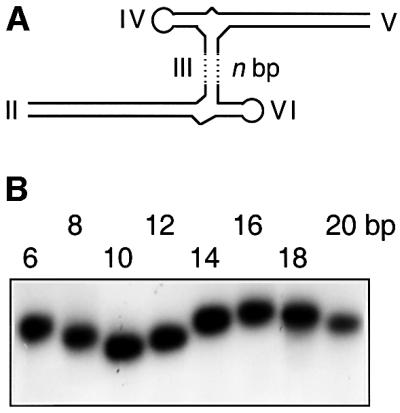
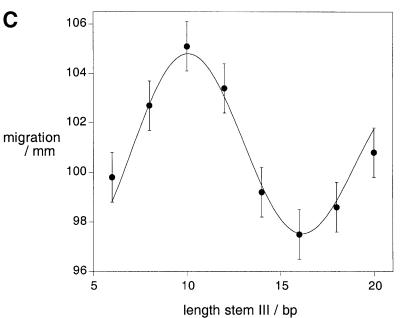
Fig. 5. Estimation of the dihedral angle between stems II and V using gel electrophoresis. (A) This analysis employed a series of variant ribozymes comprising elongated helices II and V, and short helices IV and VI, and a perfectly base paired helix III that varied in length from 6 to 20 bp, constructed from transcribed RNA. Changing the length of the central helix III would have the effect of altering the angle between the two three-way junctions, and thus the dihedral angle between helices II and V. It would be expected that electrophoretic mobility would depend on this angle. (B) Relative electrophoretic mobility of the species in polyacrylamide. Autoradiograph of the radioactive species as a function of the length of helix III. Note the sinusoidal modulation of mobility. (C) Plot of electrophoretic mobility as a function of the length of helix III. The points are the experimentally measured mobilities, with the error bars indicating the uncertainty in estimating the centres of the bands. These data have been fitted to Equation 3 using a simple geometric model from which we can calculate the end-to-end distance for each length of stem III. The dihedral angle for any chosen stem III length can be calculated from this analysis.
As the dihedral angle between helices II and V changes, we would anticipate a sinusoidal modulation of the electrophoretic mobility, and this is what we observe experimentally (Figure 5B). The maximum mobility occurs for the species in which the length of helix III is 10 bp. Helices II and V should be approximately co-planar and on opposite sides of stem III in this case; thus we would expect the dihedral angle between these helices to be ∼90° (i.e. 180 – 3 × 30°) in the maximally active ribozyme with a stem III of 7 bp. We can fit the data to the Lumpkin–Zimm model (Lumpkin and Zimm, 1982), where the mobility (µ) is proportional to the square of the end-to-end distance according to:
where L is the contour length of the molecule and hx is the component of the end-to-end vector h in the direction of the electric field. The brackets indicate an average over an ensemble of configurations. Using a simple geometric model of the ribozyme, we obtain a good fit to the experimental data (Figure 5C), from which we calculate a dihedral angle for the ribozyme with a helix III of 7 bp of ∼80°. We have extended this analysis using molecular graphics to generate an ensemble representation of the molecules used in the electrophoretic experiment. A graphical search of torsional space around the axis of helix III enabled us to compare the end-to-end distances of the model with those derived from the electrophoretic mobility and Equation 3. The best agreement of the measured end-to-end distances for all lengths of helix III gave an extrapolated dihedral angle of 70° for a 7 bp helix, in reasonable agreement with the value from the simple geometric model.
A three-dimensional global model of the 3–4–5 junction was created, based on the experimental comparative gel electrophoresis and relative FRET efficiencies (Figure 6B). This model reflects the experimentally determined relative directions of the helices, but not the detailed arrangement at the centre of the junction which presently is unknown. We also took our previous model for the 2–3–6 junction (Figure 6C) (Lafontaine et al., 2001a), and the two junctions were then brought together through a common 7 bp helix III (without including a bulged nucleotide) to produce a model for the ribozyme (Figure 6D and E). Note that this model lacks any explicit representation of the A718, A652 or A725 bulges, or the A730 loop.
The probable location of the substrate stem–loop
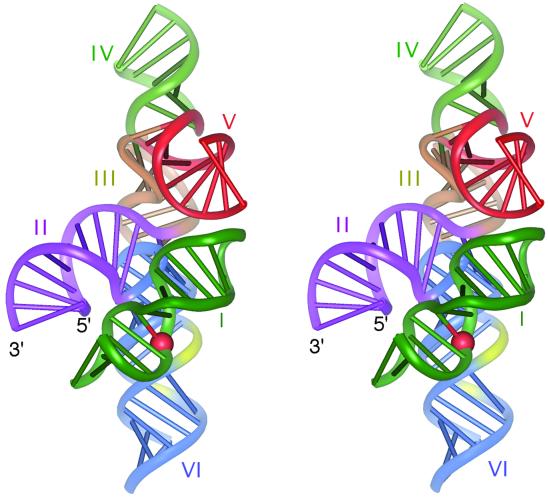
Having deduced the global shape of the five-helix ribozyme in the presence of Mg2+ ions, it remains to deduce the position of the substrate when bound to it. The structure of the substrate stem–loop has been determined in two forms by NMR (Michiels et al., 2000; Flinders and Dieckmann, 2001), although it has been suggested that the secondary structure becomes rearranged on binding to the substrate (Andersen and Collins, 2000). The location of the substrate relative to the ribozyme is subject to two constraints. First, in the cis-acting form, the 3′ end of the substrate stem is linked via three formally unpaired bases to the 3′ end of stem II, and indeed may form a three-way junction with a third helix that can form with the 5′ and 3′ extensions of the VS sequence (Jones et al., 2001). Secondly, Rastogi et al. (1996) have provided evidence for a long-range tertiary interaction between the substrate loop and that of stem V; this explains why the ribozyme activity is sensitive to the length of stem V as noted above. The most acceptable way to satisfy these two constraints is by locating the substrate in the cleft between helices II and VI, while making the loop–loop interaction with stem V. We have modelled this interaction by manually docking the structure of helix I taken from the NMR structure of Flinders and Dieckmann (2001) (PDB entry 1HWQ) into our model of the five-helix ribozyme, in such a way as to allow the substrate 3′ end to be in close proximity to the 5′ end of helix 2 and the loop region to be in close proximity to the end of helix 5 (Figure 7). At the present time, this model is essentially illustrative, and should not be taken to be a well-defined molecular structure.
Fig. 7. A parallel-eye stereographic representation of the interaction between the VS ribozyme and its substrate stem–loop. The structure of the ribozyme is the model of the five-helix structure shown in Figure 6. An NMR-derived structure of the substrate stem–loop (Flinders and Dieckmann, 2001) has been docked into the ribozyme model, such that the open end could be connected to the end of helix II, and the loop is close to that of helix V. The scissile phosphate of the substrate is highlighted spacefilling in red. The location of the A730 loop is coloured yellow; note that within the constraints imposed on the docking, it is straightforward to get a close juxtaposition between the cleavage site of the substrate and the A730 loop of helix VI. The colour coding in this model is the same as that used in Figure 6A.
Helix VI contains the A730 loop, which we previously have identified as the probable active site of the ribozyme (Lafontaine et al., 2001b). In the present absence of a structure for this loop, it has not been modelled explicitly. Nevertheless, a juxtaposition of the location of this loop and the cleavage position in the substrate is readily accommodated in the model. This is consistent with the ability to observe activity when stem–loop I is reconnected via its 5′ end to the 3′ end of stem II (Andersen and Collins, 2000). Moreover, we have been able to observe cleavage of the substrate when attached to the end of helix VI via a 5-uridine tether (D.A.Lafontaine and D.M.J.Lilley, unpublished data). Recently, Hiley and Collins (2001) have shown hydroxyl radical protection in helix II, and in helix VI at the A730 loop, consistent with the model.
The angle between helices II and V affects the activation energy for cleavage
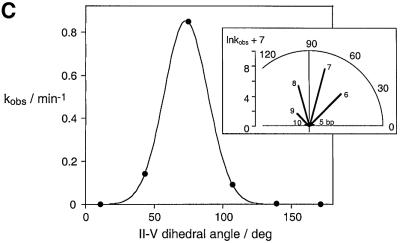
According to our model, the substrate stem–loop makes simultaneous interactions with the lower cleft, defined by helices II and VI, and the upper section via the loop–loop interaction with stem V. This interaction should therefore be strongly affected by the dihedral angle between stems II and V, i.e. by the length of helix III that connects the two junctions. We have tested this by measuring the rate of trans cleavage using a series of ribozymes in which a perfectly base paired helix III was varied in length from 4 to 10 bp (Figure 8); the rates are given in Table I. It can be seen that the optimal length is 7 bp, which has almost the same activity as the natural 6 bp plus bulged adenine (A718) as noted above. Addition or removal of a single base pair reduced the activity by 10-fold, and larger insertions or deletions reduced the activity by at least another order of magnitude. When the data are plotted on a logarithmic scale, it is seen that increases in length are tolerated a little better than reductions.
Fig. 8. Variation of cleavage activity with the length of helix III. (A) The rate of cleavage of substrate in trans was measured for a series of modified ribozymes in which the length of a perfectly base paired stem III varied from 6 to 10 bp. (B) The products of ribozyme cleavage for a 10 min incubation with each of the variant ribozymes. It can be seen that the position of cleavage is the same for each ribozyme, but the extent of reaction during the fixed time depends strongly on the length of helix III. Track 1, natural sequence ribozyme; tracks 2–7, ribozymes with helix III lengths of 5–10 bp, respectively. (C) Plot of the rate of cleavage (kobs shown by filled circles) catalysed by the variant ribozymes as a function of the stem II–V dihedral angle estimated by electrophoretic analysis (Figure 5). The insert shows a polar plot, where the lengths of the lines are proportional to ln kobs. These are also labelled with the corresponding length (bp) of helix III. It can be seen that there is a relatively narrow range of dihedral angle over which the ribozyme is active; centred on 73° (i.e. corresponding to a length of helix III of close to 7 bp). The data have been fitted (line) to a model described in the text, where it is assumed that the difference in activation energy arises from the energy required to deform the structure to the optimal angle.
On the basis of our structural model for the ribozyme, we conclude that the probable origin of the differences in rate is the additional activation energy that will be required to readjust the dihedral angle so that the substrate can be accommodated productively. Thus the differences in activation energy arise from torsional strain in stem III to a first approximation, and we have therefore fitted our observed rate constants to an equation of the form:
kobs = C·exp(–F·ΔθD2/RT)(4)
where F is a torsional force constant and ΔθD is the displacement from the optimal dihedral angle. This simple model gives a reasonable fit to the experimental data (R = 0.99999). It could be improved by allowing the force constant to vary with the length of helix III, but is adequate for the small displacements considered. The correspondence between the model and the observed cleavage rates is further support for the model for the interaction between the ribozyme and substrate.
Interaction between the substrate and the active site of the ribozyme
We propose that the substrate stem–loop interacts with the five-helix ribozyme by docking between helices II and VI, while making a tertiary loop–loop contact with stem V. This multipoint attachment is consistent with the sensitivity of the observed cleavage rate on the length of helix III, i.e. on the relative dispositions of the upper and lower sections of the ribozyme.
Modelling suggests that by placing the substrate in this position, it can be readily docked with the A730 loop (Figure 7), which we have proposed previously to be an important component of the active site. This region of the ribozyme is extremely sensitive to mutation; almost every change of sequence within this loop leads to a large reduction in cleavage activity. Any change of A756 in particular leads to a 1000-fold reduction in the rate of cleavage, while leaving substrate binding and the conformation of the ribozyme unaffected (Lafontaine et al., 2001b). In contrast, the sequence of the rest of helix VI is relatively unimportant, and the section beyond the A730 loop can be deleted almost completely with very little loss of activity. The functional significance of the A730 loop is also consistent with results of ethylnitrosourea and phosphorothioate interference, manganese rescue experiments (Sood et al., 1998) and base modification experiments (Beattie and Collins, 1997). We expect that an intimate association between the A730 loop and that of the substrate will create the local environment in which catalysis can proceed. This situation is comparable with that observed in the hairpin ribozyme, where the active form of the ribozyme is created by an interaction between two internal loops of RNA (Murchie et al., 1998; Walter et al., 1998; Rupert and Ferré-D’Amaré, 2001). A756 is a strong candidate as a participant in nucleobase catalysis, but this would require an environment in which its pKA is raised by several units. It is conceivable that this might occur within the environment of the juxtaposed loops. This interaction might also create binding sites for participating metal ions, and facilitate conformational transitions that accelerate the reaction.
Thus our present view is that the VS ribozyme undergoes metal ion-induced folding, driven by the two three-way helical junctions, into a form in which it can bind the substrate stem–loop to create an intimate association with the A730 loop. This close interaction between the two loops creates the local environment in which the transesterification reaction is accelerated.
Materials and methods
RNA synthesis and preparation of RNA constructs
Ribo-oligonucleotides were synthesized using phosphoramidite chemistry (Beaucage and Caruthers, 1981). Synthesis and purification were performed as described in Bassi et al. (1995). Junctions were prepared by incubating stoichiometric amounts of the three appropriate RNA oligonucleotides in 90 mM Tris-borate pH 8.3, 25 mM NaCl for 10 min at 80°C, followed by slow cooling. The hybridized species were electrophoresed in a polyacrylamide gel at 4°C for 22 h at 120 V. The buffer system contained 90 mM Tris–borate pH 8.3 and 25 mM NaCl, and was recirculated at >1 l/h. Fluorescent junctions were visualized by exposure to a Dark Reader transilluminator (Clare Chemical Research). The bands were excised and the RNA was electroeluted into 8 M ammonium acetate and recovered by ethanol precipitation.
Transcription of RNA
RNA was transcribed using T7 RNA polymerase (Milligan et al., 1987) from double-stranded DNA templates. Templates for transcription of ribozymes were made by recursive PCR from synthetic DNA oligonucleotides. RNA was purified by electrophoresis in 8 or 20% polyacrylamide gels containing 7 M urea. RNA was recovered from crushed gel slices by elution in water at 4°C overnight. Eluted RNA was filtered, recovered by ethanol precipitation and dissolved in water.
Analysis of ribozyme cleavage
Ribozyme cleavage reactions were performed using trace concentrations (∼1 nM) of radioactively, 5′-32P-labelled substrate and a large excess of ribozyme (1 µM). Cleavage buffer contained 50 mM Tris–HCl pH 8.0, 10 mM MgCl2, 25 mM KCl and 2 mM spermidine. Substrate and ribozyme were incubated individually in cleavage buffer at 37°C for 15 min and then mixed to initiate the reaction. Aliquots of 2 µl were removed at different times, and quenched by addition of 8 µl of 95% formamide, 20 mM EDTA, 0.05% xylene cyanol FF and 0.05% bromophenol blue. Substrate and product were separated by electrophoresis in a 20% polyacrylamide gel containing 7 M urea. They were quantified by exposure to a storage phosphor screen and imaging (Fuji). Data were fitted to single exponential functions by non-linear regression analysis (Kalaidagraph). Ribozyme and substrate strands were made by transcription. All transcribed RNA species begin with a 5′-GCG sequence in order to minimize 5′ heterogeneity (Stage-Zimmermann and Uhlenbeck, 1998). Cleavage experiments were based on the following ribozyme–substrate sequences (written 5′ to 3′), with changes where indicated in the text: substrate, GCGCGAAGGGCGUCGUCGCCCCGA; ribozyme, GCGGUAGUAAGCAGGGAACUCACCUCCAAUUUCAGUACUGAAAUUGUCGUAGCAGUUGACUACUGUUAUGUGAUUGGUAGAGGCUAAGUGACGGUAUUGGCGUAAGUCAGUAUUGCAGCACAGCACAAGCCCGCUUGCGAGAAU.
Variant ribozymes were also generated in which the length of helix III was altered, by making the changes (sequences of 5′ strand): 5 bp, helix III = CCACC; 6 bp, delete A718; 7 bp, complement A718; 8 bp, helix III = CUCGUACC; 9 bp, helix III = CUCGCUACC; 10 bp, helix III = CUCGACUACC.
Comparative gel electrophoresis
Nine ribo-oligonucleotides were prepared by transcription, of which the three longest ones had the sequences: gcgaagcgacaggaaccucgagucuagacGCGGACUCACCUCCAAUUUCACGCgcauuuccucgagguuccugucgcuucgc, gcgaagcgacaggaaccucgaggaaaugcGCGUGAAAUUGUCGUAGCAGCGCgaagcuucucgagguuccugucgcuucgc and gcgaagcgacaggaa ccucgagaagcuucGCGCUGCUAUGUGAUUGGUGAGUCCGCgucuagacucgagguuccugucgcuucgc. The remaining six oligonucleotides had 29 nucleotides deleted from the 5′ or 3′ termini (sections written in lower case above). These were hybridized in appropriate combinations to generate the species having a single shortened arm of 11 bp. Radioactively 5′-32P-labelled junctions were electrophoresed in 10% polyacrylamide in recirculated 90 mM Tris–borate pH 8.3 containing either 1 mM EDTA or 3 mM MgCl2, and subjected to autoradiography.
The dihedral angle between helices II and V was determined by comparing the electrophoretic mobility of species as a function of the length of helix III. For each species, two ribo-oligonucleotides were prepared by transcription, and hybridized to generate each construct. For the species with a stem III of 6 bp, these had the sequences: GCGACACACGGACAAGCAAUGUGUGACGUAAUAUGGAGGGAACUCACCUCCACGUCUUCGGACGUGUCGUAGCAGUUGGGUCUUAAGUUGAACAGUACAUGCAGCGC, and GCGCUGCAUGUA CUGUUCAACUUAAGACCCAACUGUUAUGUGAUUGGUGAGGCUGUGUUCGCACAGCACAAGCCCUCCAUAUUACGUCACACAUUGCUUGUCCGUGUGUCGC. The helix III for the different species had the sequences (5′ strand) CUCACC, CUCGCACC, CUCGAGCACC, CUCGAGAGCACC, CUCGAGCGAGCACC, CUCGAGCGUGAGCACC, CUCGAGCGUGUGAGCACC or CUCGAGCGUACG UGAGCACC. The constructs were electrophoresed in 10% polyacrylamide in recirculated 90 mM Tris–borate pH 8.3 containing 5 mM MgCl2.
Fluorescence spectroscopy
Fluorescence spectroscopy was performed on an SLM-Aminco 8100 fluorimeter, and spectra were corrected for lamp fluctuations and instrumental variations as described in Bassi et al. (1997). Excitation and emission polarizers were crossed at 54.74°. EFRET was measured using the acceptor normalization method (Murchie et al., 1989; Clegg, 1992) described in Lilley (2000). Junctions 3–4–5 with arm lengths of 11 bp were derived from the sequences (deoxyribonucleotides underlined): CCGGACUCACCUCCAAUUUCACGG, CCGUGAAAUUGUCGUAGCAGUGG and CCACUGCUAUGUGAUUGGUGAGUCCGG.
Molecular modelling
Molecular generation, visualization and manipulation were carried out using InsightII (Accelrys) and X-PLOR (Brünger, 1992) implemented on a Silicon Graphics Octane workstation. The coordinates of the model of the VS ribozyme can be obtained by sending a request to d.g.norman@dundee.ac.uk.
Acknowledgments
Acknowledgements
We thank our colleagues Tim Wilson, Christian Hammann and Franke Coste for discussion, Cancer Research UK and BBSRC for financial support, and EMBO and the Canadian Institutes of Health Research for fellowships awarded to D.A.L.
References
- Andersen A.A. and Collins,R.A. (2000) Rearrangement of a stable RNA secondary structure during VS ribozyme catalysis. Mol. Cell, 5, 469–478. [DOI] [PubMed] [Google Scholar]
- Bassi G., Møllegaard,N.E., Murchie,A.I.H., von Kitzing,E. and Lilley,D.M.J. (1995) Ionic interactions and the global conformations of the hammerhead ribozyme. Nature Struct. Biol., 2, 45–55. [DOI] [PubMed] [Google Scholar]
- Bassi G.S., Murchie,A.I.H., Walter,F., Clegg,R.M. and Lilley,D.M.J. (1997) Ion-induced folding of the hammerhead ribozyme: a fluorescence resonance energy transfer study. EMBO J., 16, 7481–7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie T.L. and Collins,R.A. (1997) Identification of functional domains in the self-cleaving Neurospora VS ribozyme using damage selection. J. Mol. Biol., 267, 830–840. [DOI] [PubMed] [Google Scholar]
- Beattie T.L., Olive,J.E. and Collins,R.A. (1995) A secondary-structure model for the self-cleaving region of Neurospora VS RNA. Proc. Natl Acad. Sci. USA, 92, 4686–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucage S.L. and Caruthers,M.H. (1981) Deoxynucleoside phosphor amidites—a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett., 22, 1859–1862. [Google Scholar]
- Brünger A.T. (1992) X-PLOR (version 3.1) Manual. Yale University Press.
- Buzayan J.M., Gerlach,W.L. and Bruening,G. (1986) Non-enzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature, 323, 349–353. [Google Scholar]
- Clegg R.M. (1992) Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol., 211, 353–388. [DOI] [PubMed] [Google Scholar]
- Epstein L.M. and Gall,J.G. (1987) Self-cleaving transcripts of satellite DNA from the newt. Cell, 48, 535–543. [DOI] [PubMed] [Google Scholar]
- Ferré-D’Amaré A.R., Zhou,K. and Doudna,J.A. (1998) Crystal structure of a hepatitis delta virus ribozyme. Nature, 395, 567–574. [DOI] [PubMed] [Google Scholar]
- Flinders J. and Dieckmann,T. (2001) A pH controlled conformational switch in the cleavage site of the VS ribozyme substrate RNA. J. Mol. Biol., 308, 665–679. [DOI] [PubMed] [Google Scholar]
- Forster A.C. and Symons,R.H. (1987) Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell, 49, 211–220. [DOI] [PubMed] [Google Scholar]
- Förster T. (1948) Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys., 2, 55–75. [Google Scholar]
- Gordon P.M., Sontheimer,E.J. and Piccirilli,J.A. (2000) Metal ion catalysis during the exon-ligation step of nuclear pre-mRNA splicing: extending the parallels between the spliceosome and group II introns. RNA, 6, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner,K., Marsh,T., Pace,N. and Altman,S. (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell, 35, 849–857. [DOI] [PubMed] [Google Scholar]
- Hertel K.J., Peracchi,A., Uhlenbeck,O.C. and Herschlag,D. (1997) Use of intrinsic binding energy for catalysis by an RNA enzyme. Proc. Natl Acad. Sci. USA, 94, 8497–8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley S.L. and Collins,R.A. (2001) Rapid formation of a solvent-inaccessible core in the Neurospora Varkud satellite ribozyme. EMBO J., 20, 5461–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F.D., Ryder,S.P. and Strobel,S.A. (2001) An efficient ligation reaction promoted by a Varkud satellite ribozyme with extended 5′- and 3′-termini. Nucleic Acids Res., 29, 5115–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell J.C., Saville,B.J., Mohr,S., Kuiper,M.T., Sabourin,J.R., Collins,R.A. and Lambowitz,A.M. (1995) The VS catalytic RNA replicates by reverse transcription as a satellite of a retroplasmid. Genes Dev., 9, 294–303. [DOI] [PubMed] [Google Scholar]
- Koizumi M. and Ohtsuka,E. (1991) Effects of phosphorothioate and 2-amino groups in hammerhead ribozymes on cleavage rates and Mg2+ binding. Biochemistry, 30, 5145–5150. [DOI] [PubMed] [Google Scholar]
- Lafontaine D.A., Norman,D.G. and Lilley,D.M.J. (2001a) Structure, folding and activity of the VS ribozyme: importance of the 2–3–6 helical junction. EMBO J., 20, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine D.A., Wilson,T.J., Norman,D.G. and Lilley,D.M.J. (2001b) The A730 loop is an important component of the active site of the VS ribozyme. J. Mol. Biol., 312, 663–674. [DOI] [PubMed] [Google Scholar]
- Lilley D.M.J. (2000) Analysis of the global conformation of branched RNA species by a combined electrophoresis and fluorescence approach. Methods Enzymol., 317, 368–393. [DOI] [PubMed] [Google Scholar]
- Lilley D.M.J., Clegg,R.M., Diekmann,S., Seeman,N.C., von Kitzing,E. and Hagerman,P. (1995) Nomenclature Committee of the International Union of Biochemistry: a nomenclature of junctions and branchpoints in nucleic acids. Recommendations 1994. Eur. J. Biochem., 230, 1–2. [DOI] [PubMed] [Google Scholar]
- Lumpkin O.J. and Zimm,B.H. (1982) Mobility of DNA in gel electrophoresis. Biopolymers, 21, 2315–2316. [DOI] [PubMed] [Google Scholar]
- Michiels P.J.A., Schouten,C.H.J., Hilbers,C.W. and Heus,H.A. (2000) Structure of the ribozyme substrate hairpin of Neurospora VS RNA: a close look at the cleavage site. RNA, 6, 1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J.F., Groebe,D.R., Witherall,G.W. and Uhlenbeck,O.C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res., 15, 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie A.I.H., Clegg,R.M., von Kitzing,E., Duckett,D.R., Diekmann,S. and Lilley,D.M.J. (1989) Fluorescence energy transfer shows that the four-way DNA junction is a right-handed cross of antiparallel molecules. Nature, 341, 763–766. [DOI] [PubMed] [Google Scholar]
- Murchie A.I.H., Thomson,J.B., Walter,F. and Lilley,D.M.J. (1998) Folding of the hairpin ribozyme in its natural conformation achieves close physical proximity of the loops. Mol. Cell, 1, 873–881. [DOI] [PubMed] [Google Scholar]
- Nakano S., Chadalavada,D.M. and Bevilacqua,P.C. (2000) General acid–base catalysis in the mechanism of a hepatitis delta virus ribozyme. Science, 287, 1493–1497. [DOI] [PubMed] [Google Scholar]
- Nissen P., Hansen,J., Ban,N., Moore,P.B. and Steitz,T.A. (2000) The structural basis of ribosome activity in peptide bond synthesis. Science, 289, 920–930. [DOI] [PubMed] [Google Scholar]
- Norman D.G., Grainger,R.J., Uhrin,D. and Lilley,D.M.J. (2000) The location of cyanine-3 on double-stranded DNA; importance for fluorescence resonance energy transfer studies. Biochemistry, 39, 6317–6324. [DOI] [PubMed] [Google Scholar]
- Perrotta A.T., Shih,I. and Been,M.D. (1999) Imidazole rescue of a cytosine mutation in a self-cleaving ribozyme. Science, 286, 123–126. [DOI] [PubMed] [Google Scholar]
- Pley H.W., Flaherty,K.M. and McKay,D.B. (1994) Three-dimensional structure of a hammerhead ribozyme. Nature, 372, 68–74. [DOI] [PubMed] [Google Scholar]
- Rastogi T. and Collins,R.A. (1998) Smaller, faster ribozymes reveal the catalytic core of Neurospora VS RNA. J. Mol. Biol., 277, 215–224. [DOI] [PubMed] [Google Scholar]
- Rastogi T., Beattie,T.L., Olive,J.E. and Collins,R.A. (1996) A long-range pseudoknot is required for activity of the Neurospora VS ribozyme. EMBO J., 15, 2820–2825. [PMC free article] [PubMed] [Google Scholar]
- Rupert P.B. and Ferré-D’Amaré,A.R. (2001) Crystal structure of a hairpin ribozyme–inhibitor complex with implications for catalysis. Nature, 410, 780–786. [DOI] [PubMed] [Google Scholar]
- Saville B.J. and Collins,R.A. (1990) A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell, 61, 685–696. [DOI] [PubMed] [Google Scholar]
- Saville B.J. and Collins,R.A. (1991) RNA-mediated ligation of self-cleavage products of a Neurospora mitochondrial plasmid transcript. Proc. Natl Acad. Sci. USA, 88, 8826–8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W.G., Finch,J.T. and Klug,A. (1995) The crystal structure of an all-RNA hammerhead ribozyme: a proposed mechanism for RNA catalytic cleavage. Cell, 81, 991–1002. [DOI] [PubMed] [Google Scholar]
- Sharmeen L., Kuo,M.Y., Dinter-Gottlieb,G. and Taylor,J. (1988) Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J. Virol., 62, 2674–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood V.D. and Collins,R.A. (2001) Functional equivalence of the uridine turn and the hairpin as building blocks of tertiary structure in the Neurospora VS ribozyme. J. Mol. Biol., 313, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Sood V.D., Beattie,T.L. and Collins,R.A. (1998) Identification of phosphate groups involved in metal binding and tertiary interactions in the core of the Neurospora VS ribozyme. J. Mol. Biol., 282, 741–750. [DOI] [PubMed] [Google Scholar]
- Stage-Zimmermann T.K. and Uhlenbeck,O.C. (1998) Hammerhead ribozyme kinetics. RNA, 4, 875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol H., Buzayan,J.M., Feldstein,P.A., Eckstein,F. and Bruening,G. (1990) Two autolytic processing reactions of a satellite RNA proceed with inversion of configuration. Nucleic Acids Res., 18, 1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N.G., Hampel,K.J., Brown,K.M. and Burke,J.M. (1998) Tertiary structure formation in the hairpin ribozyme monitored by fluorescence resonance energy transfer. EMBO J., 17, 2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter N.G., Burke,J.M. and Millar,D.P. (1999) Stability of hairpin ribozyme tertiary structure is governed by the interdomain junction. Nature Struct. Biol., 6, 544–549. [DOI] [PubMed] [Google Scholar]
- Welch J.B., Duckett,D.R. and Lilley,D.M.J. (1993) Structures of bulged three-way DNA junctions. Nucleic Acids Res., 21, 4548–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yean S.-L., Wuenschell,G., Termini,J. and Lin,R.-J. (2000) Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature, 408, 881–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.-Y., Wilson,T.J., Maxwell,K. and Lilley,D.M.J. (2000) The folding of the hairpin ribozyme: dependence on the loops and the junction. RNA, 6, 1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]