Abstract
Particles of most virus species accurately package a single genome, but there are indications that the pleomorphic particles of parainfluenza viruses incorporate multiple genomes. We characterized a stable measles virus mutant that efficiently packages at least two genomes. The first genome is recombinant and codes for a defective attachment protein with an appended domain interfering with fusion-support function. The second has one adenosine insertion in a purine run that interrupts translation of the appended domain and restores function. In that genome, a one base deletion in a different purine run abolishes polymerase synthesis, but restores hexameric genome length, thus ensuring accurate RNA encapsidation, which is necessary for efficient replication. Thus, the two genomes are complementary. The infection kinetics of this mutant indicate that packaging of multiple genomes does not negatively affect growth. We also show that polyploid particles are produced in standard infections at no expense to infectivity. Our results illustrate how the particles of parainfluenza viruses efficiently accommodate cargoes of different volume, and suggest a mechanism by which segmented genomes may have evolved.
Keywords: cargo volume/envelope/measles virus/pleomorphic particles/polyploid viruses
Introduction
Virus particles, with a few exceptions, incorporate only one genome. The structure of the particles is a determinant of the accuracy with which this genome is packaged. Icosahedral symmetry imposes stringent constraints on genome size. Viruses with helical nucleocapsid and a pleomorphic envelope have relaxed constraints on genome length. Particle sedimentation and ultraviolet inactivation studies indicate that the pleomorphic particles of parainfluenza viruses often incorporate more than one genome (Hosaka et al., 1966; Dahlberg and Simon, 1969). More over, orthomyxoviruses may incorporate more than one haploid set of segments (Enami et al., 1991).
Measles virus (MV) is a paramyxovirus that produces extremely pleomorphic particles with diameters ranging from 120 to >300 nm (Waterson, 1965; Nakai and Imagawa, 1969; Nakai et al., 1969; Lund et al., 1984). Since the envelope, comprising the membrane, the fusion (F) and hemagglutinin (H) glycoproteins and the membrane-associated matrix (M) protein, is ∼20 nm wide, the cargo space of the particles may vary from 3 × 105 to >107 nm3. Considering that the diameter and length of the ribonucleoprotein complex (RNP) are 20 and 1000 nm, respectively, particles should be able to accommodate from one to >30 genomes. However, since the infectivity to particle ratio in MV is low, it has been questioned whether the large particles are functional.
The genetic material of paramyxoviruses consists of RNA of negative polarity (Lamb and Kolakofsky, 2001). This RNA is covered by the nucleocapsid protein N, the phosphoprotein P, which serves as an assembly as well as a polymerase cofactor, and the RNA polymerase L. The 15 894-nucleotide MV genome is organized into six contiguous, non-overlapping transcription units that are separated by short untranslated regions and code for the six structural viral proteins in the order 5′-N-P-M-F-H-L-3′ (positive strand) (Radecke and Billeter, 1995; Griffin, 2001). In addition, the P cistron encodes the two non-structural proteins C and V from overlapping open reading frames (ORFs), accessed by different mechanisms. Ribo somal choice during translational initiation results in translation of the C protein from an overlapping ORF downstream of the P AUG codon (Bellini et al., 1985). The V protein shares the N-terminal 231 amino acids with the P protein. However, the 68 C-terminal amino acids are translated from a different reading frame accessed by the cotranscriptional insertion of a non-templated G residue, a process referred to as mRNA editing (Thomas et al., 1988; Cattaneo et al., 1989).
As shown for the model paramyxovirus Sendai (SeV), each N protein in the nucleocapsid tightly associates with precisely six bases (Egelman et al., 1989; Calain and Roux, 1993). Presumably as a consequence of this interaction, the genomes of most paramyxoviruses are replicated efficiently only if their length is a multiple of six bases (Murphy and Parks, 1997). It has long been thought that the basis for this phenomenon, dubbed the ‘rule of six’, is that an exact nucleotide-N match at the RNA 3′-OH end (3′-OH congruence) is required for recognition of an active replication promoter. However, recent findings suggest that some nucleotide positions relative to N (N phase context) may be critical, at least in some regions of the genome and anti-genome promoters (Vulliemoz and Roux, 2001). Analysis of the replication of SeV mini-genome analogs whose lengths were not multiples of six showed that hexameric genome length can be restored by the insertion or deletion of purines at the P editing site during anti-genome synthesis. It was therefore suggested that this site may serve the dual function of editing and genome length correction (Hausmann et al., 1996).
Comparing the strict requirements set by the rule of six, MV tolerates large variations in total genome length. Up to three additional reading frames have been introduced into the MV genome and were found to be stably propagated (L.Hangartner, T.Cornu and M.Billeter, unpublished data). In addition, different specificity domains, as diverse as a growth factor or a single-chain antibody, were successfully displayed on the H protein and conferred entry through targeted receptors (Schneider et al., 2000; Hammond et al., 2001).
The genome length flexibility, the envelope versatility, and the facts that paramyxovirus genomes do not integrate or recombine (Toyoda et al., 1989; Pringle, 1991) and that the MV live attenuated vaccine is extremely safe (Palumbo et al., 1992; Moss et al., 1999) make this virus an attractive candidate as a therapeutic vector. In fact, even the unmodified vaccine strain MV Edmonston has been approved for a clinical phase I trial in patients with refractory B-cell lymphoma (A.Fielding, personal communication). The rationales for this study are the observation that several lymphoma cases regressed completely after coincidental MV infection (Bluming and Ziegler, 1971; Taqi et al., 1981) and the demonstration that MV Edmonston can cause regression of human lymphoma and myeloma xenografts in mice (Grote et al., 2001; Peng et al., 2001).
To extend the scope of MV-based cytoreductive therapy, additional targets are being considered and several different specificity domains are being displayed on the H protein. However, we show here that not all displayed domains are well tolerated and that high selective pressure compromises the stability of certain vectors. We confirm that MV can package multiple genomes, as has previously been suggested. Moreover, we show that a virus that incorporates at least two genomes propagates with comparable efficiency to an unmodified control virus.
Results
CD4 domains appended to the H protein interfere with MV propagation
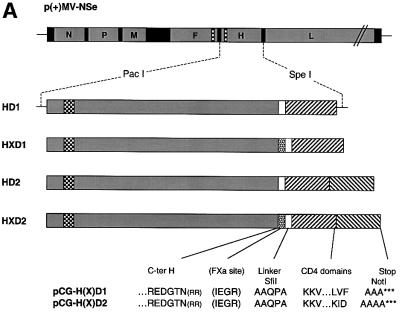
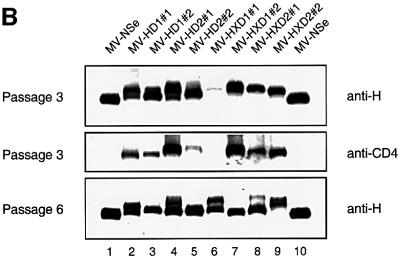
We generated hybrid proteins in which the coding region for either the first or the first two CD4 domains were fused to the C-terminus of the H coding region (HD1 and HD2; Figure 1A). Two additional hybrid proteins were constructed in which a factor Xa protease cleavage site was added before the linker (HXD1 and HXD2). In a func tional assay based on syncytium formation (Cathomen et al., 1995), it was found that the H/CD4 hybrid proteins are not able to efficiently support fusion (data not shown). Nevertheless, we transferred the corresponding genes to the infectious cDNA clone p(+)MV-NSe (Figure 1A, top panel) in place of the standard H gene. All four viruses were recovered 10–14 days after transfection. Western blot analysis of infected cells showed a mixture of proteins with the expected molecular weight, but also smaller proteins that were not recognized by an anti-CD4 antibody (Figure 1B, compare the top with the middle panel). The fraction of shorter proteins increased with the number of passages (Figure 1B, compare top and bottom panels).
Fig. 1. Genomic structure and protein expression of recombinant MV. (A) Plasmid p(+)MV-NSe encoding the MV anti-genome (top), PacI–SpeI fragments used for subcloning (center), and amino acid sequences (one-letter code) of the junctions between the H protein ectodomain and the CD4 domains (bottom). Coding regions of the six MV cistrons are represented by gray boxes, intergenic regions by solid black boxes, transmembrane domains by checkered boxes, the FXa cleavage site by a dotted box, the flexible linker by a white box and the CD4 domains by hatched boxes. Arginine residues in parentheses were deleted to avoid the possibility of introducing an undesired furin cleavage site (Schneider et al., 2000). (B) Western blot analysis of H/CD4 hybrid protein expression. Vero cells infected with the recombinant viruses were lysed at passage 3 (top and center) or passage 6 (bottom). The proteins were seperated by SDS–PAGE and probed with either an H-specific antiserum (top and bottom) or with a CD4-specific antiserum (center).
To analyze whether the genome of the recovered viruses still corresponded to the cDNAs that were initially transfected, we performed RT–PCR on RNA extracted from infected cells. We found six different variants with point mutations (Figure 2A, boxed nucleotides) resulting in stop codons (TGA, TAG or TAA). Remarkably, two mutants with a single insertion leading to a reading frame shift were also selected (Figure 2A, large boxes). All the stop mutations were located in the first CD4 domain and occurred before the second cysteine (Cys, line 5), which would allow structural stabilization of the CD4 domain. The complete upstream H sequence was unaffected.
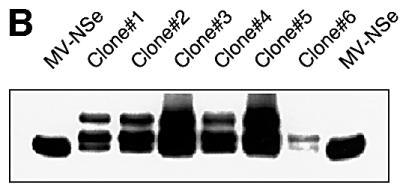
Fig. 2. Stop mutations in the recombinant viral genomes and propagation of the insertion mutant MV-HXD2#2. (A) The genomes of recovered recombinant viruses were analyzed by RT–PCR of infected Vero cell RNA. For simplicity, all the mutations found in the different clones of MV-HD1, MV-HD2, MV-HXD1 and MV-HXD2 are represented on the sequence of MV-HXD2. The last 15 codons of the H ORF are shown, connected to CD4 domains 1 and 2 by a four-amino-acid flexible linker and the five-amino-acid factor Xa cleavage site (bold). CD4D1, sequence of domain 1 of CD4; CD4D2, sequence of domain 2 of CD4. The premature stop codon resulting from the single nucleotide insertion is underlined. (B) Western blot analysis of MV-HXD2#2 clones. Six distinct syncytia from a MV-HXD2#2 infection were picked and grown on fresh Vero cells. Cell lysates were fractionated by SDS–PAGE and proteins were detected with an anti-H cytoplasmic tail antiserum. (C) Genomic sequence of the A insertion site (I–IV) and the P-editing site (V) in MV-HXD2#2 at different passages as determined by RT–PCR of RNA extracted from infected cells (I–III) and from purified viral particles (IV and V).
Mutant MV-HXD2#2 producing two H proteins is stable
In most cases, virus populations consisting only of the mutated variants were rapidly selected (Figure 1B, compare passage 3 with passage 6, lanes 2–8). Remarkably, this was not the case with mutant MV-HXD2#2 (lane 9), which maintained two H proteins with slow migration characteristics even at passage 6. Genome analysis of MV-HXD2#2 by RT–PCR on infected cells at passages 3 and 6 showed the same heterogeneity (Figure 2C, I and II). The electropherogram is homogeneous until an A6 sequence (positions 142–147 in Figure 2C, I), but becomes heterogeneous after this sequence due to an A nucleotide insertion at this position in a sizeable fraction of the cDNAs.
To investigate the basis for this heterogeneity, we selected virus clones and determined the electrophoretic mobility of the expressed proteins and the sequence of the corresponding genomes. H proteins from all six plaque-purified clones displayed the same migration pattern on western blotting (Figure 2B) and the corresponding genes had identical sequences (one example is shown in Figure 2C, III). After 15 passages, six of which were performed by plaque purification, a mixture of hybrid and shortened H proteins was still detected in all clones. This was observed by western blotting and confirmed by RT–PCR of RNA produced in infected cells (data not shown). Thus, in infected cells derived from single plaques, two populations of H mRNA existed, one corresponding to the parental sequence and accounting for the longer, non-functional H protein, and one with an A insertion accounting for the shorter protein.
Since in MV and other paramyxoviruses one or more pseudo-templated G residues are inserted by polymerase stuttering at a predetermined position near a polypurine stretch (Cattaneo et al., 1989), and since a similar polypurine stretch occurs near the new A-insertion site, we hypothesized that polymerase stuttering occurring in the CD4 coding region accounted for the insertion of As in a sizeable fraction of the mRNA. Since this hypothesis predicts that genomic RNA has a homogeneous sequence, we purified virus particles from the cell supernatant over a sucrose cushion and examined the encapsidated genomes by RT–PCR. Whereas the editing site in P showed no insertion (Figure 2C, V), proving that we had indeed obtained a pure virus particle preparation, an approximate 1:1 ratio of parental to mutated sequence was observed in the CD4 domain (Figure 2C, IV). We therefore concluded that cotranscriptional polymerase stuttering did not account for our observations.
Two populations of genomes of hexameric length coexist in MV-HXD2#2 infections
We then hypothesized that two populations of genomes may coexist, such that MV-HXD2#2 infectivity consists of a mixture of genomes coding for a mutated but functional H protein, and of genomes coding for the engineered elongated H protein. To examine the possibility that different genomes might be packaged as concatemers, we attempted to amplify the potential genome junction region by RT–PCR on purified virions (Figure 3A). However, the expected 1932 bp fragment was not obtained, showing that no concatemers are formed (data not shown).
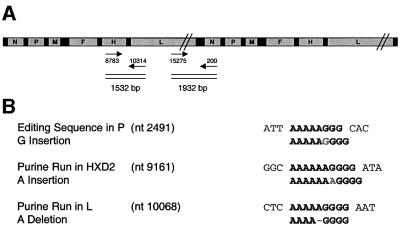
Fig. 3. RT–PCR analysis of MV-HXD2#2. (A) The MV-HXD2#2 genome is depicted as the putative concatemer. The 1932 bp fragment that would have resulted from RT–PCR on purified particles of a virus carrying concatemeric genomes, and the 1532 bp fragment amplified to investigate linkage between the A insertion in the CD4 domain and the A deletion in the L reading frame, are indicated. Numbers near the arrows correspond to the positions in the 16 398-nucleotide MV-HXD2 genome. (B) Sequence of the polypurine stretches at the P editing site, the insertion site and the deletion site. The mutated sequence is shown below the parental sequence.
Assuming that in MV, as in the related paramyxovirus Sendai (Calain and Roux, 1993), only genomes of hexameric length are efficiently packaged, it seemed likely that in the genomes with an A addition, a compensating deletion would occur. We first examined the intergenic sequences, since a deletion there would not affect any of the viral proteins; however, no mutations were present in these regions (data not shown). We then tested whether a mutation had occurred in one of the three proteins forming the nucleocapsid N, P or L, which could explain altered editing or packaging properties. Indeed we discovered an A deletion in a purine run 334 nucleotides downstream of the L protein start codon (Figure 3B). This deletion leads to the re-establishment of hexameric genomic length, but due to the frameshift and premature stop codon also results in a truncated and therefore non-functional L fragment of only 127 amino acids.
To investigate the frequency with which the A deletion at the beginning of the L reading frame was linked to the A insertion in the CD4 domain coding region, we amplified a 1532 bp fragment comprising the end of the H/CD4 reading frame and the beginning of the L reading frame by RT–PCR on RNA extracted from purified virus particles (Figure 3A). Single cDNAs were cloned into a PCR-TOPO vector. Of the 24 clones sequenced, 16 fragments carried both the insertion and the deletion sequences, whereas eight fragments carried the parental sequence. Three clones had a double instead of a single A insertion. Fragments with only either the insertion or the deletion sequence were not observed. Thus, after 15 passages, two distinct genomes constituted the majority of the viral RNA: the parental genomes with a functional L polymerase, and the genomes possessing both a nucleotide addition in H and a compensating deletion in L. Both genomes can be efficiently replicated because their length in bases is a multiple of six.
MV-HXD2#2 packages multiple genomes into single particles
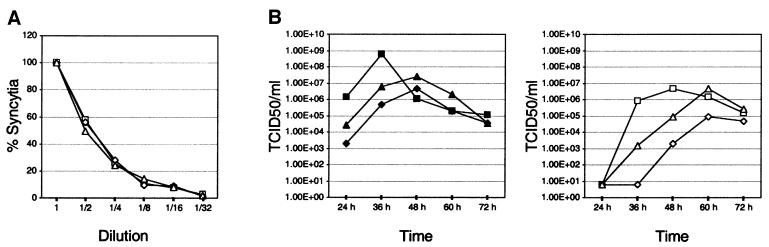
A mixture of genomes can be propagated either by packaging multiple RNAs in single particles or by co-infection of the same cells with particles carrying single genomes. In the first case, one hit kinetics of infection is expected, and in the second case, multiple hit kinetics. To distinguish between these two possible mechanisms we performed a dilution assay. Vero cells were infected with 1:2 serial dilutions of MV-HXD2#2 and the two control viruses MV-NSe and MV-eGFP [the latter expresses enhanced green fluorescent protein (eGFP) from an additional transcription unit], and syncytia were counted. Since the likelihood of co-infection lessens upon dilution of the virus, MV-HXD2#2 would be expected to lose infectivity more rapidly than the control viruses if co-infection was a necessary event for its propagation. This was not the case, however. In fact, MV-HXD2#2 exhibited very similar behavior to MV-NSe and MV-eGFP upon dilution, indicating that it follows single hit kinetics (Figure 4A). A different dilution assay, in which increasing numbers of cells were infected with a constant amount of virus [100 plaque-forming units (p.f.u.)], produced the same result (data not shown).
Fig. 4. Growth kinetics of MV-HXD2#2. (A) Dilution assay with MV-NSe (squares), MV-eGFP (diamonds) and MV-HXD2#2 (triangles). Vero cells in six-well plates were infected with 1:2 serial dilutions of the respective viruses starting at a concentration of 200 p.f.u./ml. Syncytia were counted 5 days post-infection. The number of syncytia in the well containing undiluted virus was set to 100% and relative percentages were calculated. (B) Time course of cell-associated (closed symbols, left panel) and released (open symbols, right panel) virus production in Vero cells infected with MV-HXD2#2 (triangles), or the control viruses MV-NSe (squares) and MV-eGFP (diamonds). Cells were infected at a m.o.i. of 0.03 and incubated at 37°C for the times indicated. Viral titers were determined by 50% end-point dilution.
We also examined the time course of released and cell-associated virus production. MV-HXD2#2 showed no significant differences in growth kinetics or end titer when compared with MV-NSe and MV-eGFP (Figure 4B). We therefore conclude that the packaging of multiple genomes does not occur at the expense of efficient replication.
A significant fraction of functional MV particles with unmodified glycoproteins is polyploid
Functional H protein is present at the virion surface as disulfide-linked dimers, which may associate into tetramers (Plemper et al., 2000). Oligomerization of parental and modified H proteins may destabilize these interactions and produce an undiscriminating envelope of irregular structure, as observed in certain influenza virus glycoprotein mutants (Jin et al., 1997). To investigate whether the ability to package multiple genomes is unique to the mutant MV-HXD2#2, or if it also applies to MV particles with unmodified envelope glycoproteins, we infected Vero cells simultaneously with MV-eGFP and a MV expressing red fluorescent protein (MV-DsRed1). Syncytia that expressed eGFP as well as DsRed1, showing that they had been infected with both types of virus, were picked, propagated on fresh Vero cells and the number of ‘yellow’ (eGFP- and DsRed1-expressing) syncytia was counted (Figure 5, passage 1). To measure the proportion of syncytia coexpressing eGFP and DsRed1 as a result of infection by viral aggregates rather than individual particles, MV-eGFP and MV-DsRed1 were mixed and incubated on ice for 1 h prior to infection (Figure 5, control). Thirteen percent of syncytia in passage 1 were yellow compared with 8% in the aggregation control, suggesting that infection with individual viral particles carrying both genomes accounted for part of the yellow syncytia.
Fig. 5. Co-infection assay with MV-eGFP and MV-DsRed1. Vero cells were simultaneously infected with MV-eGFP and MV-DsRed1, and syncytia expressing both eGFP and DsRed1 were passaged. The percentage of syncytia expressing only eGFP (gray), only DsRed1 (black), and both eGFP and DsRed1 (white) was determined. The total number of syncytia counted for each passage is given in parentheses. In the aggregation control experiment, MV-eGFP and MV-DsRed1 were mixed and incubated on ice for 1 h prior to infection.
If all particles were randomly packaging two or more genomes, however, at least 50% yellow progeny would be expected. Since it is known that a cell contains distinct replication centers (Cathomen et al., 1998), it is conceivable that genomes originating from the same replication center are more likely to be co-packaged, resulting in a relatively high fraction of green only or red only syncytia. We therefore hypothesized that repeated passaging of yellow syncytia would increase the probability of originating mixed replication centers, and thus enhance the fraction of viruses with mixed genomes. The number of yellow syncytia indeed increased with the number of passages, reaching 46% in passage 9 (Figure 5). However, the fraction of yellow progeny strongly fluctuated between passages and between the progeny of different plaques, presumably reflecting the relative location of replication centers within each individual syncytium. Nevertheless, these findings suggest that MV particles composed of only standard viral proteins can package multiple genomes.
Discussion
Serendipity bestowed an intriguing MV mutant that gained an additional form of the attachment protein and maintained it stably. Nucleic acid analysis of this mutant not only revealed the molecular basis of the observed phenotype, but also proved that polyploid MV particles are functional, and provided insights into the determinants of efficient ribonucleocapsid replication.
Hexameric genome length
It has been assumed that the MV genome, like that of other paramyxoviruses, is efficiently replicated only if its length is a multiple of six. The rule of six has been discovered in spontaneously arising and slightly altered mini-genomes of the copy-back type of the paramyxovirus SeV (Calain and Roux, 1993), and shown to hold also for artificially constructed mini-replicons of the internal deletion type of MV (Sidhu et al., 1995). For constructed mini-replicons of SeV, it was shown that the P gene mRNA editing site, a polypurine run into which one or more pseudo-templated G residues are cotranscriptionally added, can also serve to re-establish hexameric mini-replicon genome length, a process termed ‘genome length correction’ (Hausmann et al., 1996). However, it appears unlikely that this mechanism is of any relevance to infections since P protein expression would be severely altered, and possibly abolished, for such corrected genomes. In fact, experiments with full-length MV genomes show that mutants with a G insertion at the editing site are not viable unless P is exogenously provided (Schneider et al., 1997a).
Extensive studies of the replication of SeV mini-replicons suggested that genome length correction differs from the G insertions occurring during mRNA synthesis in three important aspects: (i) purines (A and G residues) can be deleted as well as inserted; (ii) it can only be detected at a significant frequency if the constructed mini-genome is not of hexamer length; and (iii) only the A6G3 sequence and no additional upstream sequences are required (Hausmann et al., 1996). No genome length correction has been observed at the polyadenylation sites (a 4- to 7-nucleotide run of template U residues) in the SeV mini-replicon system (Kolakofsky et al., 1998).
We show here that a MV in which two CD4 domains had been appended to the H protein, thereby disrupting its fusion-support function, either acquired stop codons or gained an A or a G residue at an A6G4 sequence in the first CD4 domain. The insertion leads to a reading frame shift and therefore interrupted translation of the appended domain. Hexameric genome length was restored through an A deletion at an A5G4 sequence at the beginning of the L reading frame, leading to a truncated polymerase. This demonstrates that not only the P editing site, but also other polypurine runs, are prone to polymerase stuttering. That stuttering in full-length MV genome replication is not restricted to A6G3 sequences is further confirmed by our findings that one of the analyzed clones carried an A deletion at a G2A5 sequence in the first CD4 domain, and that two other constructs of non-hexameric length compensated this defect by inserting an A residue at an A4 and A5 polyadenylation site, respectively (data not shown).
It cannot be expected that the rule of six is an absolute requirement for genome replication. Among the order Mononegavirales, only the subfamily Paramyxovirinae appear to follow it rigorously, and as suggested by mini-replicons of simian virus 5 (Murphy and Parks, 1997), the genus rubulavirus may adhere less strictly to the rule than the genera respirovirus and morbillivirus. Nevertheless, in these genera, the encapsidation process appears to be extremely accurate over the entire genome, leading to ribonucleocapsids without a single gap between the N molecules. Transiently arising mutant genomes not adhering to the rule, but selected by virtue of re-established protein functions, are likely to be quickly displaced by secondary mutants in which the rule is re-established.
Polyploid viruses
The mutant genome of MV-HXD2#2 codes for an H protein with restored fusion-support function and also for a non-functional polymerase. Thus, it can replicate only in the presence of the original recombinant genome coding for an intact polymerase. Indeed, the two genomes cannot be separated by repeated rounds of plaque purification or by purification of virus particles. The fact that MV-HXD2#2 displays growth kinetics similar to the control viruses MV-NSe and MV-eGFP demonstrates that at least two genomes can efficiently be packaged in the same particle at no expense to viral replication. The altered hemagglutinin does not account for this observation since co-infection with the two viruses MV-eGFP and MV-DsRed1, in which all the viral structural proteins are unmodified, yields a significant fraction of progeny syncytia coexpressing both eGFP and DsRed1. This indicates that many MV particles generated in cultivated cells are polyploid.
Most DNA and positive-strand RNA viruses have icosahedral capsids and therefore a clearly defined cargo space that not only allows them to package only one genome, but also sets precise upper and lower limits for the length of it (Flint et al., 2000). Retroviruses are exceptional in their characteristic of incorporating two copies of their positive-strand RNA genome. Negative-strand RNA viruses have a helical nucleocapsid and a more versatile envelope that does not impose tight constraints on total genome length. Paramyxoviruses are more pleomorphic than most other negative-strand RNA viruses, and early evidence suggested that a significant fraction of their particles is multiploid (Simon, 1972). Other negative-strand RNA virus families may also be able to package more than one genome. It has been shown that orthomyxoviruses can incorporate more than one set of their genome segments (Enami et al., 1991). Filoviruses, while maintaining a constant diameter, are highly variable in length (Sanchez et al., 2001) and may therefore be polyploid. However, peak infectivity of filoviruses has been associated with relatively short filaments (Kiley et al., 1982). Rhabdovirus bullet-like particles, on the contrary, are relatively constant in length. In fact, it has been shown that recombinant vesicular stomatitis virus (VSV) particles containing an additional CD4 reading frame were ∼18% longer than wild-type virions, reflecting the additional length of the nucleocapsid (Schnell et al., 1996). Length and volume measurements suggest that VSV particles package only one genome.
In summary, particles of several negative-strand RNA viruses may have a uniquely versatile envelope structure, enabling them to accommodate cargoes of different size at no expense to infectivity. Certain viruses like MV, which combine the ability to propagate by cell fusion with poly ploidy, are expected to be very permissive in propagating defective genomes. Indeed, heterogeneous populations of defective genomes have been characterized in persistent MV infections in human brains (Cattaneo et al., 1988; Baczko et al., 1993). Moreover, these results suggest a mechanism by which segmented viral genomes may have evolved from non-segmented ones. This mechanism would involve the packaging of multiple genomes, followed by the reduction of redundant information, as exemplified by the alteration of a polymerase gene. Ultimately, the independent genomes will be degraded to interdependent segments.
Materials and methods
Plasmids
Expression plasmids containing the H/CD4 hybrid genes were generated by amplifying either the first domain (nucleotides 76–369) or the first two domains (nucleotides 76–591) of CD4 from pT7T-CD4 (Mebatsion et al., 1997), starting at the first codon after the 25 amino acid signal peptide. A SfiI site (underlined) was introduced by the common forward primer 5′-GACAGGCCCAGCCGGCCAAGAAAGTGGTGCTGGG-3′ and a NotI site by the reverse primers 5′-ATAAGAATGCGGCCGCGAACACTAGCAATTGCA-3′ (domain 1) and 5′-ATAAGAATGCGGCCGCTGCGTCTATTTTGAACTCCACC-3′ (domain 2). The PCR fragments were cloned into the SfiI–NotI-digested plasmids pCG-H/hIGF1-c6 (pCG-HD1 and pCG-HD2) or pCG-H/XhIGF1-c6 (pCG-HXD1 and pCG-HXD2), which had previously been used for expression of hybrid proteins and corrected so as to yield PacI–SpeI fragments that would result in genomes of hexameric length when cloned into infectious MV cDNA (Schneider et al., 2000). The plasmids obtained by inserting the hybrid H genes into PacI–SpeI-digested p(+)MV-Nse (Singh et al., 1999) were named p(+)MV-HD1, p(+)MV-HD2, p(+)MV-HXD1 and p(+)MV-HXD2.
Cells and viruses
Vero cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal calf serum (FCS). The rescue helper cell line 293-3-46 was grown in DMEM containing 10% FCS and 1.2 mg/ml G418. Viruses were propagated on Vero cells.
Transfections
For the fusion experiments, Vero cells were transfected with the F protein expression plasmid pCG-F (Cathomen et al., 1995), and either a plasmid encoding the parental H protein (pCG-H) (Cathomen et al., 1995) or the hybrid protein expression vectors using different molar ratios varying from 1:1 to 1:4. The latter had previously been determined to be the most effective for fusion activity in the closely related canine distemper virus (von Messling et al., 2001). SuperFect (Qiagen) was used as a transfection reagent, following the manufacturer’s instructions.
Recovery of recombinant viruses
293 cells were infected with MVA-T7 (Schneider et al., 1997b) at a multiplicity of infection (m.o.i.) of 0.8, and seeded into six-well plates at a density of 106 cells per well. The calcium phosphate transfection was performed using the Profection mammalian transfection system (Promega). Four micrograms of the respective anti-genomic plasmid and a set of three plasmids from which the proteins of the MV polymerase complex are expressed (2 µg of N-protein plasmid, 2 µg of P-protein plasmid and 0.5 µg of L-protein plasmid in 10 mM Tris–HCl pH 8.5) were diluted in 175 µl of double-distilled water. Then, 25 µl of 2 M CaCl2 were added to the solution followed by vortexing. This mixture was added dropwise to 200 µl of 2× HEPES-buffered saline pH 7.1 while vortexing continuously. After incubation for 30 min at room temperature, the mixture was added dropwise to the cells. The supernatant was removed the next day, and the cells were maintained in DMEM with 10% FCS for 3 days. Cells of each well were transferred to a 100-mm-diameter culture dish into which Vero cells had been seeded at 50–60% confluency. After an additional 3 days, the culture medium was replaced with DMEM lacking FCS. Nine days after transfection, the cells were expanded into 150-mm-diameter dishes and maintained in DMEM 5% FCS until syncytia were detected.
Immunoblotting
Vero cells infected with the recombinant viruses or the control virus MV-NSe were disrupted by the addition of lysis buffer (50 mM Tris pH 8.0, 62.5 mM EDTA, 0.4% deoxycholate, 1% Igepal; Sigma) supplemented with complete protease inhibitor (Roche Biochemicals) and 1 mM phenylmethylsulfonyl fluoride (PMSF), and the lysates were clarified by centrifugation at 1300 g for 10 min at 4°C. The supernatants were denatured for 15 min at 85°C in Laemmli sample buffer (Bio-Rad) containing 10% β-mercaptoethanol, fractionated on 10% SDS–poly acrylamide gels (Bio-Rad) and blotted on polyvinylidene fluoride membranes (Millipore). The membranes were blocked with 1% bovine serum albumin, 4% skim milk powder in TBST (10 mM Tris pH 8.0, 150 mM NaCl, 0.05% Tween-20), probed with either rabbit anti-H cytoplasmic tail antiserum (Cathomen et al., 1998) at 1:5000 dilution, or sheep anti-CD4 antiserum [AIDS Reagent Program (ARRRP)] at 1:1000 dilution. Following incubation with peroxidase-conjugated goat anti-rabbit (Jackson ImmunoResearch) or donkey anti-sheep IgG (Sigma), respectively, proteins were visualized by enhanced chemiluminescence (Pierce).
RT–PCR
Eighty percent confluent Vero cells on six-well plates were infected with the different viral clones expressing H/CD4 hybrid proteins and incubated at 37°C until an extensive cytopathic effect was observed. Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and reverse transcribed using Superscript II RNase H– Reverse Transcriptase (Gibco-BRL) and random primers. The region of interest was then amplified using the Expand High Fidelity PCR system (Roche Biochemicals) and specific primers. The purified PCR products were sequenced. For analysis of single RNAs, the PCR products were cloned into TOPO TA cloning vectors (Invitrogen) according to the manufacturer’s protocol prior to sequencing. To analyze RNA packaged into viral particles, two T175 flasks (Falcon) with Vero cells at 80% confluence were infected at a m.o.i. of 0.1 and incubated at 32°C until ∼90% of all nuclei were engaged in syncytia. Supernatants were collected and clarified by centrifugation at 8000 r.p.m. for 20 min at 4°C in a SA-600 rotor (Sorvall). The supernatant was transferred into a 36 ml polypropylene tube (Sorvall) and pelleted by velocity centrifugation (28 000 r.p.m. for 90 min at 4°C) through 20% sucrose onto a 60% sucrose cushion prepared in TNE buffer (10 mM Tris–HCl pH 7.5, 100 mM NaCl, 1 mM EDTA). The virus-containing interphase fraction was harvested, diluted to <30% sucrose by the addition of TNE buffer, and pelleted by 90 min centrifugation at 28 000 r.p.m. All liquid was carefully removed and the pellet was taken up in 350 µl lysate of uninfected Vero cells in RLT buffer (Qiagen) to add carrier RNA for RNA isolation, which was performed as described above for total cellular RNA.
Dilution assay
Starting with 200 p.f.u./ml, 1:2 serial dilutions of the viruses MV-NSe, MV-eGFP (Duprex et al., 1999) and MV-HXD2#2 were performed in OptiMEM. Eighty percent confluent Vero cells on six-well plates were infected with 1 ml of each dilution. Two hours after infection, all liquid was removed and the cells were overlaid with 1% SeaPlaque agarose in DMEM with 5% FCS. Five days post-infection, cells were fixed with 10% trichloroacetic acid, stained with crystal violet and syncytia were counted.
Growth curves
Vero cells (5 × 105/well) in six-well plates were infected at a m.o.i. of 0.03 with MV-NSe, MV-eGFP or MV-HXD2#2, and incubated at 37°C. Released and cell-associated virus was collected every 12 h and stored at –80°C. The 50% tissue culture infectious dose (TCID50) of the samples was determined on Vero cells.
Co-infection assay
Vero cells on six-well plates were infected simultaneously with MV-eGFP and MV-DsRed1 (W.P.Duprex, unpublished data), each at a m.o.i. of 0.05, and subsequently overlaid with 1% SeaPlaque agarose in DMEM 5% FCS. After 2–3 days, syncytia coexpressing both eGFP and DsRed1 were picked and used to infect fresh Vero cells. Agarose overlay was performed for this (passage 1) and all subsequent passages. For each passage, 12 distinct yellow (eGFP- and DsRed1-expressing) syncytia were picked and propagated. To determine the background of syncytia coexpressing eGFP and DsRed1 as a result of infection by viral aggregates, the same amounts of MV-eGFP and MV-DsRed1 as those used for the initial co-infection were mixed and incubated on ice for 1 h. This inoculum was used to infect Vero cells on a six-well plate, and the number of syncytia expressing both eGFP and DsRed1 were counted.
Acknowledgments
Acknowledgements
We thank Martin Billeter, Adele Fielding, Urs Greber and Steve Russell for critical comments on the manuscript. This work was supported by grants from the Swiss National Science Foundation, and from the Siebens and Eisenberg Foundations. The salary of M.R. was paid, in part, by the Roche Foundation and that of W.P.D. in part by the Levitt Foundation. An antiserum to CD4 was obtained through the AIDS Research and Reference Reagent Program.
References
- Baczko K. et al. (1993) Clonal expansion of hypermutated measles virus in a SSPE brain. Virology, 197, 188–195. [DOI] [PubMed] [Google Scholar]
- Bellini W.J., Englund,G., Rozenblatt,S., Arnheiter,H. and Richardson,C.D. (1985) Measles virus P gene codes for two proteins. J. Virol., 53, 908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluming A.Z. and Ziegler,J.L. (1971) Regression of Burkitt’s lymphoma in association with measles infection. Lancet, 2, 105–106. [DOI] [PubMed] [Google Scholar]
- Calain P. and Roux,L. (1993) The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol., 67, 4822–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomen T., Buchholz,C.J., Spielhofer,P. and Cattaneo,R. (1995) Preferential initiation at the second AUG of the measles virus F mRNA: a role for the long untranslated region. Virology, 214, 628–632. [DOI] [PubMed] [Google Scholar]
- Cathomen T., Naim,H.Y. and Cattaneo,R. (1998) Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol., 72, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Schmid,A., Eschle,D., Baczko,K., ter Meulen,V. and Billeter,M.A. (1988) Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell, 55, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin,K., Baczko,K. and Billeter,M.A. (1989) Measles virus editing provides an additional cysteine-rich protein. Cell, 56, 759–764. [DOI] [PubMed] [Google Scholar]
- Dahlberg J.E. and Simon,E.H. (1969) Physical and genetic studies of Newcastle disease virus: evidence for multiploid particles. Virology, 38, 666–678. [DOI] [PubMed] [Google Scholar]
- Duprex W.P., McQuaid,S., Hangartner,L., Billeter,M.A. and Rima,B.K. (1999) Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol., 73, 9568–9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelman E.H., Wu,S.S., Amrein,M., Portner,A. and Murti,G. (1989) The Sendai virus nucleocapsid exists in at least four different helical states. J. Virol., 63, 2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Sharma,G., Benham,C. and Palese,P. (1991) An influenza virus containing nine different RNA segments. Virology, 185, 291–298. [DOI] [PubMed] [Google Scholar]
- Flint J.S., Enquist,L.W., Krug,R.M., Racaniello,V.R. and Shalka,A.M. (2000) Foundations of Virology. In Principles of Virology. ASM Press, Washington, DC, pp. 16–17.
- Griffin D.E. (2001) Measles virus. In Knipe,D.M. and Howley,P.M. (eds), Fields Virology. Vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA, pp. 1401–1441.
- Grote D., Russell,S.J., Cornu,T.I., Cattaneo,R., Vile,R., Poland,G.A. and Fielding,A.K. (2001) Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood, 97, 3746–3754. [DOI] [PubMed] [Google Scholar]
- Hammond A.L., Plemper,R.K., Zhang,J., Schneider,U., Russell,S.J. and Cattaneo,R. (2001) Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcino embryonic antigen. J. Virol., 75, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann S., Jacques,J.P. and Kolakofsky,D. (1996) Paramyxovirus RNA editing and the requirement for hexamer genome length. RNA, 2, 1033–1045. [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Kitano,H. and Ikeguchi,S. (1966) Studies on the pleo morphism of HVJ virons. Virology, 29, 205–221. [DOI] [PubMed] [Google Scholar]
- Jin H., Leser,G.P., Zhang,J. and Lamb,R.A. (1997) Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J., 16, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M.P. et al. (1982) Filoviridae: a taxonomic home for Marburg and Ebola viruses? Intervirology, 18, 24–32. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Pelet,T., Garcin,D., Hausmann,S., Curran,J. and Roux,L. (1998) Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J. Virol., 72, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R.A. and Kolakofsky,D. (2001) Paramyxoviridae: the viruses and their replication. In Knipe,D.M. and Howley,P.M. (eds), Fields Virology. Vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA, pp. 1305–1340.
- Lund G.A., Tyrrell,D.L., Bradley,R.D. and Scraba,D.G. (1984) The molecular length of measles virus RNA and the structural organization of measles nucleocapsids. J. Gen. Virol., 65, 1535–1542. [DOI] [PubMed] [Google Scholar]
- Mebatsion T., Finke,S., Weiland,F. and Conzelmann,K.K. (1997) A CXCR4/CD4 pseudotype rhabdovirus that selectively infects HIV-1 envelope protein-expressing cells. Cell, 90, 841–847. [DOI] [PubMed] [Google Scholar]
- Moss W.J., Cutts,F. and Griffin,D.E. (1999) Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin. Infect. Dis., 29, 106–112. [DOI] [PubMed] [Google Scholar]
- Murphy S.K. and Parks,G.D. (1997) Genome nucleotide lengths that are divisible by six are not essential but enhance replication of defective interfering RNAs of the paramyxovirus simian virus 5. Virology, 232, 145–157. [DOI] [PubMed] [Google Scholar]
- Nakai M. and Imagawa,D.T. (1969) Electron microscopy of measles virus replication. J. Virol., 3, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Shand,F.L. and Howatson,A.F. (1969) Development of measles virus in vitro. Virology, 38, 50–67. [DOI] [PubMed] [Google Scholar]
- Palumbo P., Hoyt,L., Demasio,K., Oleske,J. and Connor,E. (1992) Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J., 11, 1008–1014. [DOI] [PubMed] [Google Scholar]
- Peng K.W., Ahmann,G.J., Pham,L., Greipp,P.R., Cattaneo,R. and Russell,S.J. (2001) Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood, 98, 2002–2007. [DOI] [PubMed] [Google Scholar]
- Plemper R.K., Hammond,A.L. and Cattaneo,R. (2000) Characterization of a region of the measles virus hemagglutinin sufficient for its dimerization. J. Virol., 74, 6485–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C.R. (1991) The genetics of paramyxoviruses. In Kingsbury,D.W. (ed.), The Paramyxoviruses. Plenum Press, New York, NY, pp. 1–39.
- Radecke F. and Billeter,M.A. (1995) Appendix: measles virus anti genome and protein consensus sequences. Curr. Top. Microbiol. Immunol., 191, 181–192. [DOI] [PubMed] [Google Scholar]
- Sanchez A., Khan,A.S., Zaki,S.R., Nabel,G.J., Ksiazek,T.G. and Peters,C.J. (2001) Filoviridae: Marburg and Ebola viruses. In Knipe,D.M. and Howley,P.M. (eds), Fields Virology. Vol. 1. Lippincott Williams & Wilkins, Philadelphia, PA, pp. 1279–1304.
- Schneider H., Kaelin,K. and Billeter,M.A. (1997a) Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology, 227, 314–322. [DOI] [PubMed] [Google Scholar]
- Schneider H., Spielhofer,P., Kaelin,K., Dotsch,C., Radecke,F., Sutter,G. and Billeter,M.A. (1997b) Rescue of measles virus using a replication-deficient vaccinia-T7 vector. J. Virol. Methods, 64, 57–64. [DOI] [PubMed] [Google Scholar]
- Schneider U., Bullough,F., Vongpunsawad,S., Russell,S.J. and Cattaneo,R. (2000) Recombinant measles viruses efficiently entering cells through targeted receptors. J. Virol., 74, 9928–9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M.J., Buonocore,L., Kretzschmar,E., Johnson,E. and Rose,J.K. (1996) Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl Acad. Sci. USA, 93, 11359–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu M.S. et al. (1995) Rescue of synthetic measles virus mini replicons: measles genomic termini direct efficient expression and propagation of a reporter gene. Virology, 208, 800–807. [DOI] [PubMed] [Google Scholar]
- Simon E.H. (1972) The distribution and significance of multiploid virus particles. Prog. Med. Virol., 14, 36–67. [PubMed] [Google Scholar]
- Singh M., Cattaneo,R. and Billeter,M.A. (1999) A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J. Virol., 73, 4823–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taqi A.M., Abdurrahman,M.B., Yakubu,A.M. and Fleming,A.F. (1981) Regression of Hodgkin’s disease after measles. Lancet, 1, 1112. [DOI] [PubMed] [Google Scholar]
- Thomas S.M., Lamb,R.A. and Paterson,R.G. (1988) Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell, 54, 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi,T., Hirota,H., Gotoh,B., Kuma,K., Miyata,T. and Nagai,Y. (1989) Newcastle disease virus evolution. II. Lack of gene recombination in generating virulent and avirulent strains. Virology, 169, 273–282. [DOI] [PubMed] [Google Scholar]
- von Messling V., Zimmer,G., Herrler,G., Haas,L. and Cattaneo,R. (2001) The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol., 75, 6418–6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliemoz D. and Roux,L. (2001) ‘Rule of six’: how does the Sendai virus RNA polymerase keep count? J. Virol., 75, 4506–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson A.P. (1965) Recent advances in the study of measles. Anglo Ger. Med. Rev., 3, 95–100. [PubMed] [Google Scholar]