Abstract
The major use of the fluorescence recovery after photobleaching (FRAP) technique is to measure the translational motion of the molecular components in various condensed media. In a conventional laser spot photobleaching experiment, a photomultiplier is used to measure the total brightness levels of the bleached region in the sample, so no spatial information can be directly obtained. In video-FRAP, a series of images after photobleaching is acquired, allowing the spatial character of the recovery to be determined; this permits direct detection of both anisotropic diffusion and flow. To utilize all of the available image data to determine the transport coefficients, a two-dimensional spatial Fourier transform analysis of the images after photobleaching was employed. The change in the transform between two time points reflects the action of diffusion during the interim. An important advantage of this method, which involves taking the ratio of image transforms at different time points, is that it does not require a specific initial condition to be created by laser photobleaching. The ability of the analysis to extract transport coefficients from computer-simulated diffusional recovery is assessed in the presence of increasing amounts of noise. Experimental data analysis from the diffusion of proteins in viscous solutions and from the diffusion of protein receptors on cell surfaces demonstrate the feasibility of the Fourier analysis to obtain transport coefficients from the video FRAP measurement.
Full text
PDF

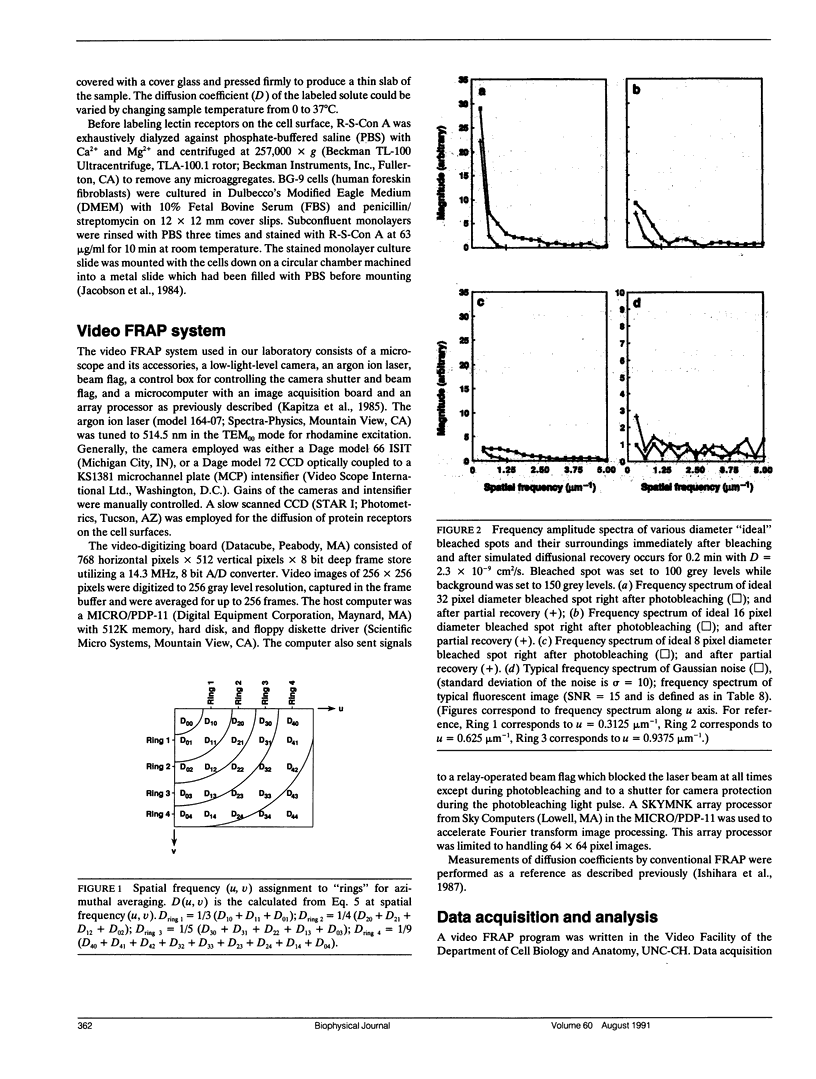

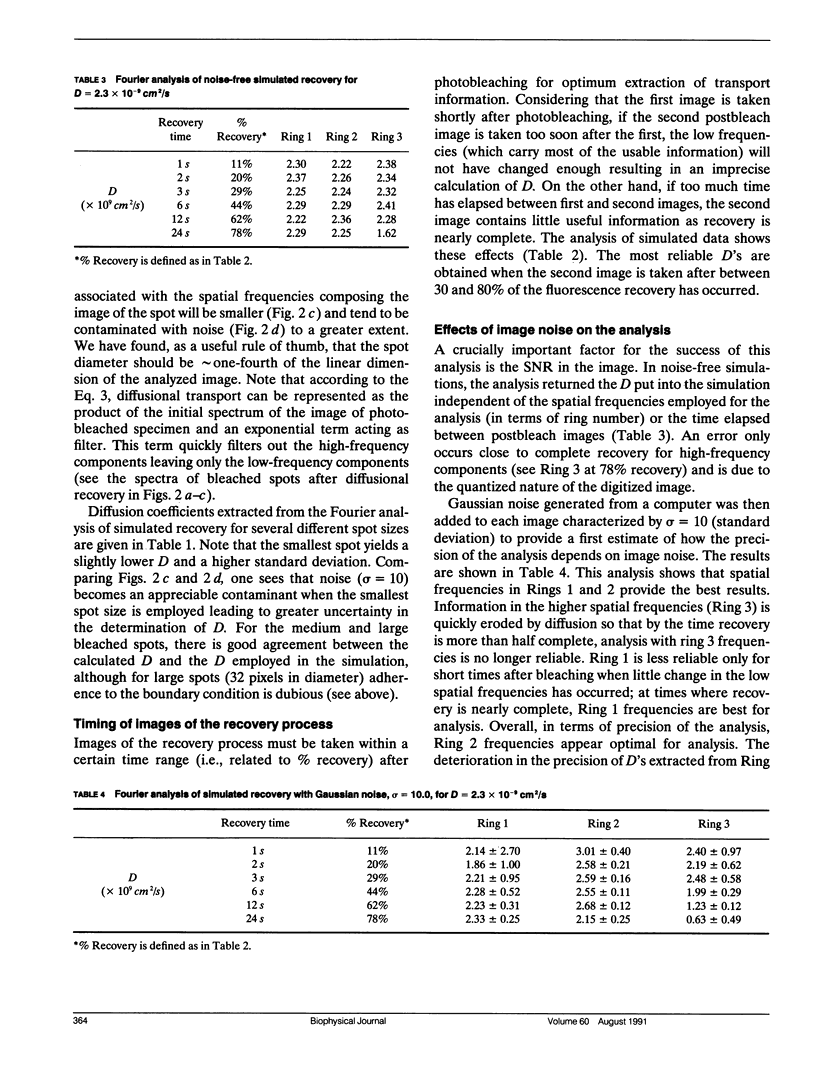
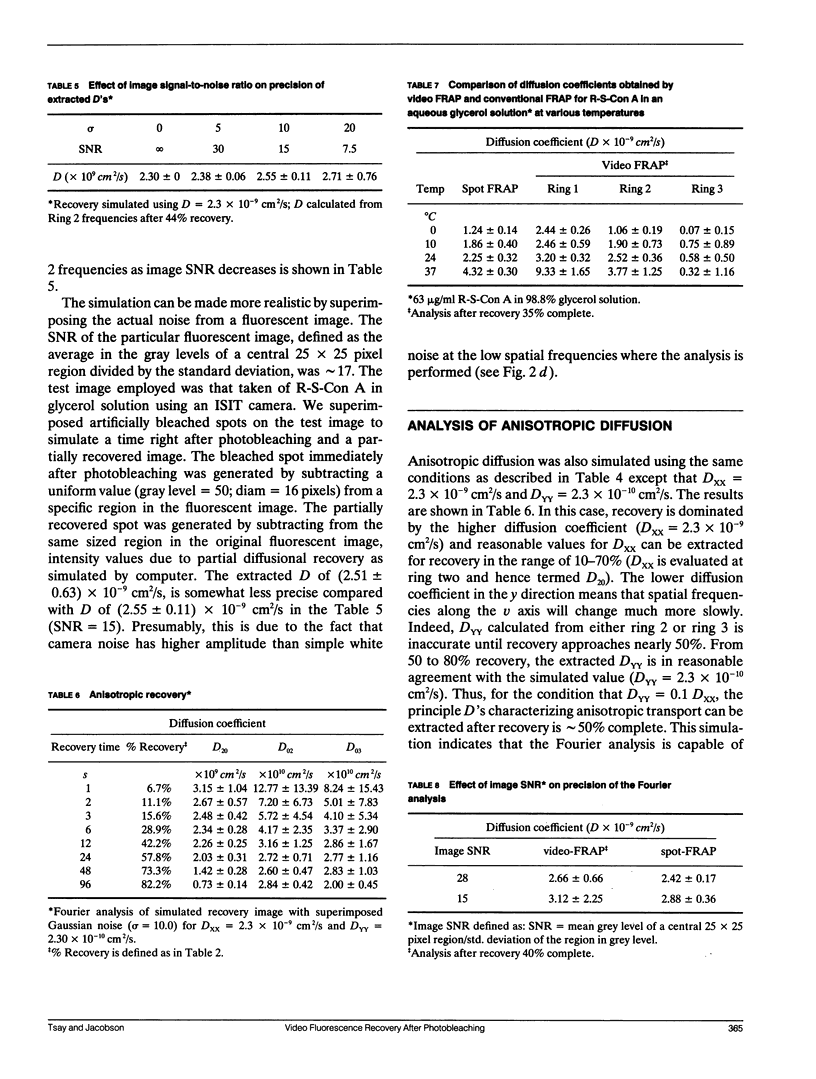



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Robert-Nicoud M., Kaufman S. J., Jovin T. M. Fluorescence digital imaging microscopy in cell biology. Science. 1985 Oct 18;230(4723):247–256. doi: 10.1126/science.4048934. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisas B. G., Leuther M. D. Fluorescence photobleaching recovery measurement of protein absolute diffusion constants. Biophys Chem. 1979 Sep;10(2):221–229. doi: 10.1016/0301-4622(79)85044-9. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Hou Y., Jacobson K. The Thy-1 antigen exhibits rapid lateral diffusion in the plasma membrane of rodent lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1290–1293. doi: 10.1073/pnas.84.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J. T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblasts: relationships to cell structure and function. J Cell Biol. 1984 Nov;99(5):1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitza H. G., McGregor G., Jacobson K. A. Direct measurement of lateral transport in membranes by using time-resolved spatial photometry. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4122–4126. doi: 10.1073/pnas.82.12.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Gustafsson M., Magnusson K. E., Jacobson K. The direction of membrane lipid flow in locomoting polymorphonuclear leukocytes. Science. 1990 Mar 9;247(4947):1229–1233. doi: 10.1126/science.2315695. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K., Lanni F., Taylor D. L. The submicroscopic properties of cytoplasm as a determinant of cellular function. Annu Rev Biophys Biophys Chem. 1988;17:369–396. doi: 10.1146/annurev.bb.17.060188.002101. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Leslie R. J., Saxton W. M., Karow M. L., McIntosh J. R. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol. 1984 Dec;99(6):2165–2174. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Smith B. A., Clark W. R., McConnell H. M. Anisotropic molecular motion on cell surfaces. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5641–5644. doi: 10.1073/pnas.76.11.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpen A. H., Pober J. S., Brown C. S., Golan D. E. Class I major histocompatibility complex proteins diffuse isotropically on immune interferon-activated endothelial cells despite anisotropic cell shape and cytoskeletal organization: application of fluorescence photobleaching recovery with an elliptical beam. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1844–1848. doi: 10.1073/pnas.85.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L. Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling. J Cell Biol. 1985 Aug;101(2):597–602. doi: 10.1083/jcb.101.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]