Abstract
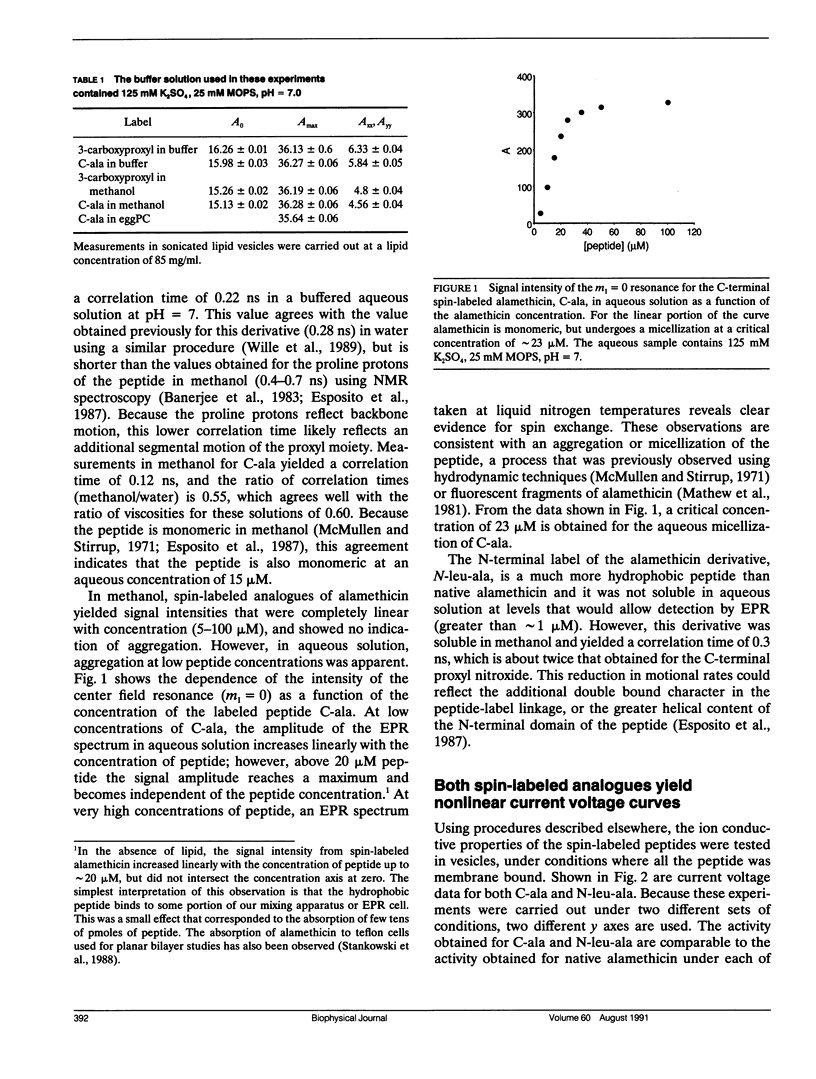
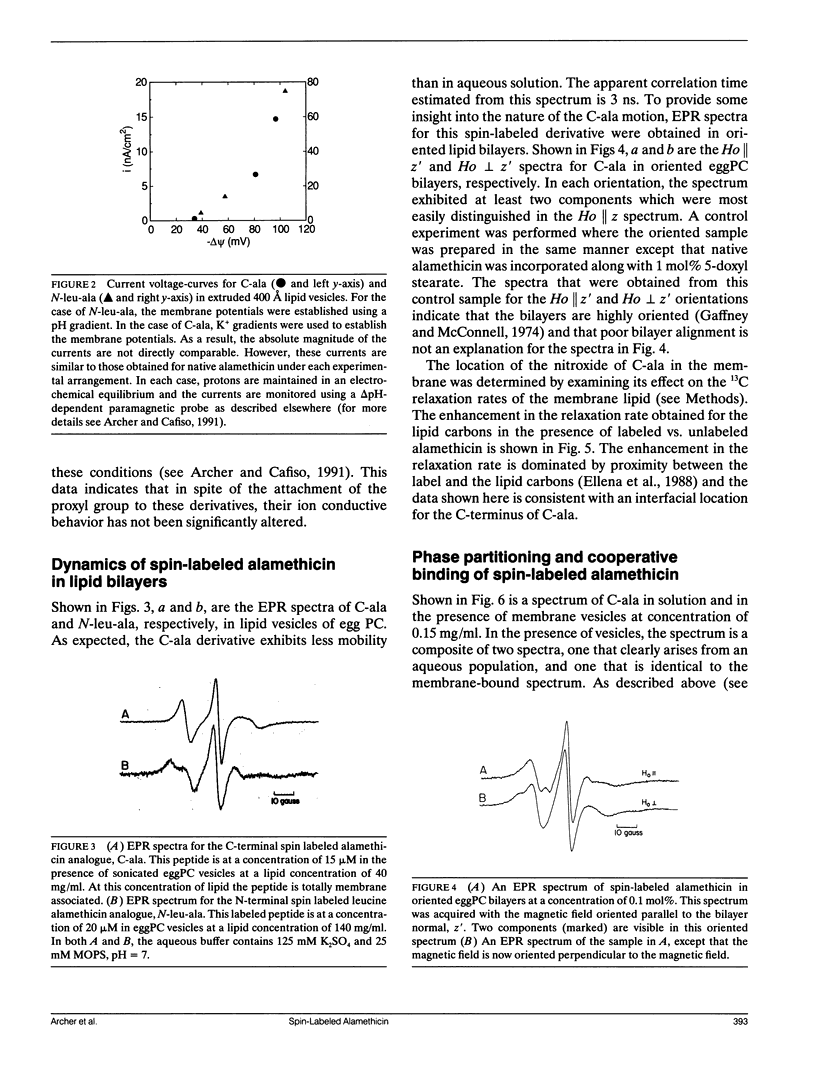
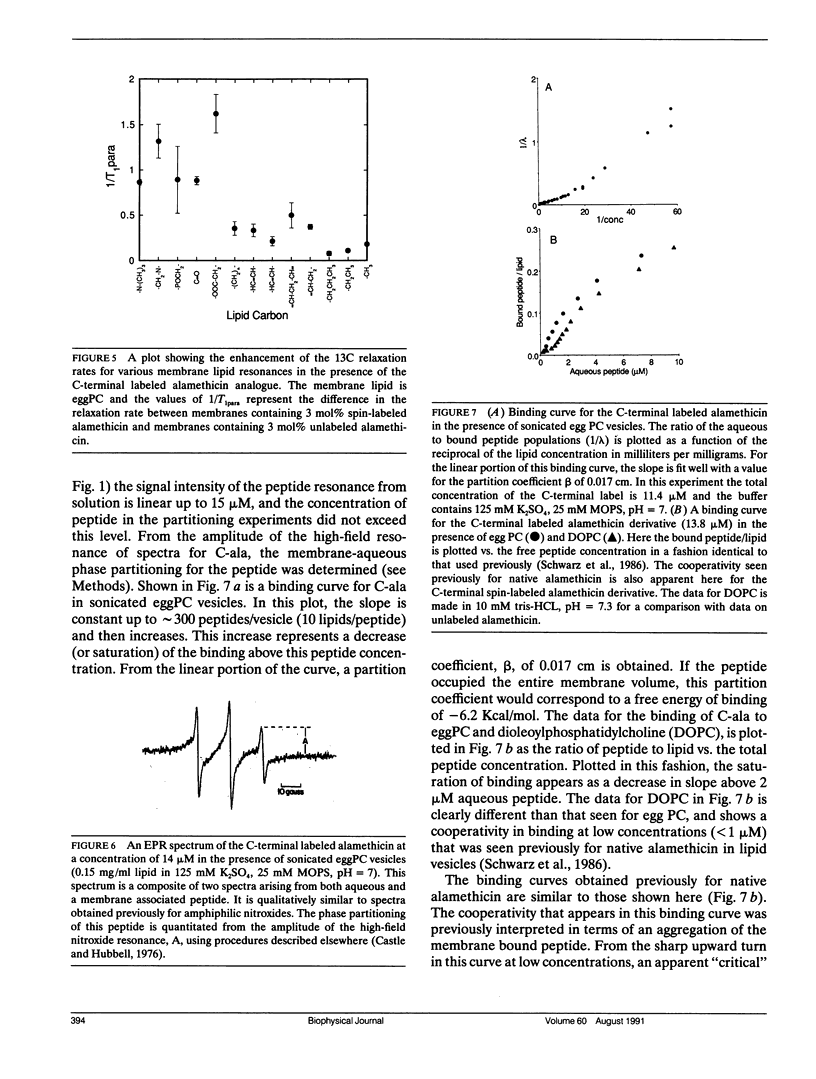
Two spin-labeled derivatives of the ion conductive peptide alamethicin were synthesized and used to examine its binding and state of aggregation. One derivative was spin labeled at the C-terminus and the other, a leucine analogue, was spin labeled at the N-terminus. In methanol, both the C and N terminal labeled peptides were monomeric. In aqueous solution, the C-terminal derivative was monomeric at low concentrations, but aggregated at higher concentrations with a critical concentration of 23 microM. In the membrane, the C-terminal label was localized to the membrane-aqueous interface using 13C-NMR, and could assume more than one orientation. The membrane binding of the C-terminal derivative was examined using EPR, and it exhibited a cooperativity seen previously for native alamethicin. However, this cooperativity was not the result of an aggregation of the peptide in the membrane. When the spectra of either the C or N-terminal labeled peptide were examined over a wide range of membrane lipid to peptide ratios, no evidence for aggregation could be found and the peptides remained monomeric under all conditions examined. Because electrical measurements on this peptide provide strong evidence for an ion-conductive aggregate, the ion-conductive form of alamethicin likely represents a minor fraction of the total membrane bound peptide.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. J., Cafiso D. S. Voltage-dependent conductance for alamethicin in phospholipid vesicles. A test for the mechanism of gating. Biophys J. 1991 Aug;60(2):380–388. doi: 10.1016/S0006-3495(91)82063-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Banerjee U., Tsui F. P., Balasubramanian T. N., Marshall G. R., Chan S. I. Structure of Alamethicin in solution. One- and two-dimensional 1H nuclear magnetic resonance studies at 500 MHz. J Mol Biol. 1983 Apr 25;165(4):757–775. doi: 10.1016/s0022-2836(83)80279-4. [DOI] [PubMed] [Google Scholar]
- Banerjee U., Zidovetzki R., Birge R. R., Chan S. I. Interaction of alamethicin with lecithin bilayers: a 31P and 2H NMR study. Biochemistry. 1985 Dec 17;24(26):7621–7627. doi: 10.1021/bi00347a019. [DOI] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Magnetic resonance spectra of membranes. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1451–1455. doi: 10.1073/pnas.72.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Estimation of transmembrane potentials from phase equilibria of hydrophobic paramagnetic ions. Biochemistry. 1978 Jan 10;17(1):187–195. doi: 10.1021/bi00594a028. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Ellena J. F., Archer S. J., Dominey R. N., Hill B. D., Cafiso D. S. Localizing the nitroxide group of fatty acid and voltage-sensitive spin-labels in phospholipid bilayers. Biochim Biophys Acta. 1988 May 9;940(1):63–70. doi: 10.1016/0005-2736(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Esposito G., Carver J. A., Boyd J., Campbell I. D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987 Feb 24;26(4):1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys J. 1986 Feb;49(2):531–540. doi: 10.1016/S0006-3495(86)83663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godici P. E., Landsberger F. R. The dynamic structure of lipid membranes. A 13C nuclear magnetic resonance study using spin labels. Biochemistry. 1974 Jan 15;13(2):362–368. doi: 10.1021/bi00699a022. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Yates J. R., 3rd, Shabanowitz J., Winston S., Hauer C. R. Protein sequencing by tandem mass spectrometry. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A., Bulfield G., Snipes W. Spin-labeled Neurospora mitochondria. Biophys J. 1970 Jul;10(7):618–629. doi: 10.1016/S0006-3495(70)86324-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew M. K., Nagaraj R., Balaram P. Fluorescent alamethicin fragments. A study of membrane activity and aqueous phase aggregation. Biochim Biophys Acta. 1981 Dec 7;649(2):336–342. doi: 10.1016/0005-2736(81)90423-5. [DOI] [PubMed] [Google Scholar]
- McMullen A. I., Stirrup J. A. The aggregation of alamethicin. Biochim Biophys Acta. 1971 Sep 14;241(3):807–814. doi: 10.1016/0005-2736(71)90008-3. [DOI] [PubMed] [Google Scholar]
- Molle G., Duclohier H., Dugast J. Y., Spach G. Design and conformation of non-Aib synthetic peptides enjoying alamethicin-like ionophore activity. Biopolymers. 1989 Jan;28(1):273–283. doi: 10.1002/bip.360280128. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Rizzo V., Stankowski S., Schwarz G. Alamethicin incorporation in lipid bilayers: a thermodynamic study. Biochemistry. 1987 May 19;26(10):2751–2759. doi: 10.1021/bi00384a015. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G., Stankowski S., Rizzo V. Thermodynamic analysis of incorporation and aggregation in a membrane: application to the pore-forming peptide alamethicin. Biochim Biophys Acta. 1986 Sep 25;861(1):141–151. doi: 10.1016/0005-2736(86)90573-0. [DOI] [PubMed] [Google Scholar]
- Snipes W., Cupp J., Cohn G., Keith A. Electron spinal resonance analysis of the nitroxide spin label 2,2,6,6-tetramethylpipidone-N-oxyl (Tempone) in single crystals of the reduced Tempone matrix. Biophys J. 1974 Jan;14(1):20–32. doi: 10.1016/s0006-3495(74)85900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankowski S., Schwarz G. Lipid dependence of peptide-membrane interactions. Bilayer affinity and aggregation of the peptide alamethicin. FEBS Lett. 1989 Jul 3;250(2):556–560. doi: 10.1016/0014-5793(89)80795-1. [DOI] [PubMed] [Google Scholar]
- Stankowski S., Schwarz U. D., Schwarz G. Voltage-dependent pore activity of the peptide alamethicin correlated with incorporation in the membrane: salt and cholesterol effects. Biochim Biophys Acta. 1988 Jun 7;941(1):11–18. doi: 10.1016/0005-2736(88)90208-8. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Mehlhorn R. J., Macey R. I. Amine spin probe permeability in sonicated liposomes. J Membr Biol. 1989 Jul;109(1):53–64. doi: 10.1007/BF01870790. [DOI] [PubMed] [Google Scholar]
- Wille B., Franz B., Jung G. Location and dynamics of alamethicin in unilamellar vesicles and thylakoids as model systems. A spin label study. Biochim Biophys Acta. 1989 Nov 17;986(1):47–60. doi: 10.1016/0005-2736(89)90271-x. [DOI] [PubMed] [Google Scholar]


