Abstract
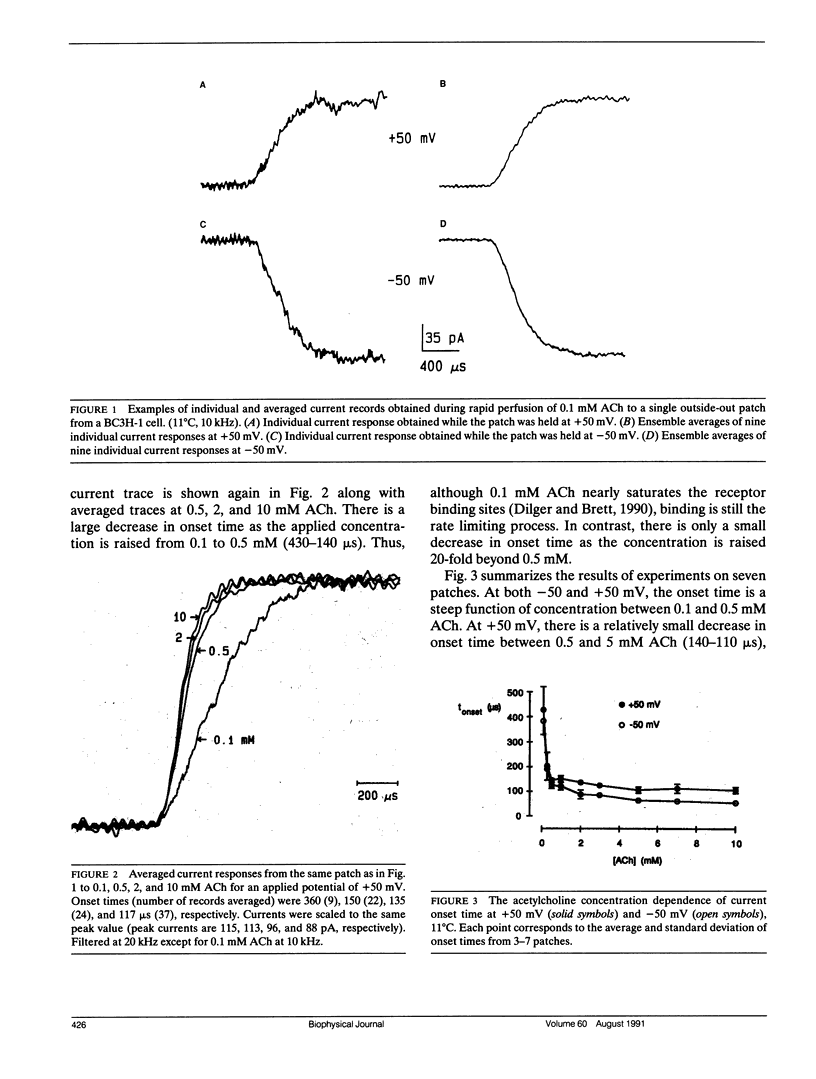
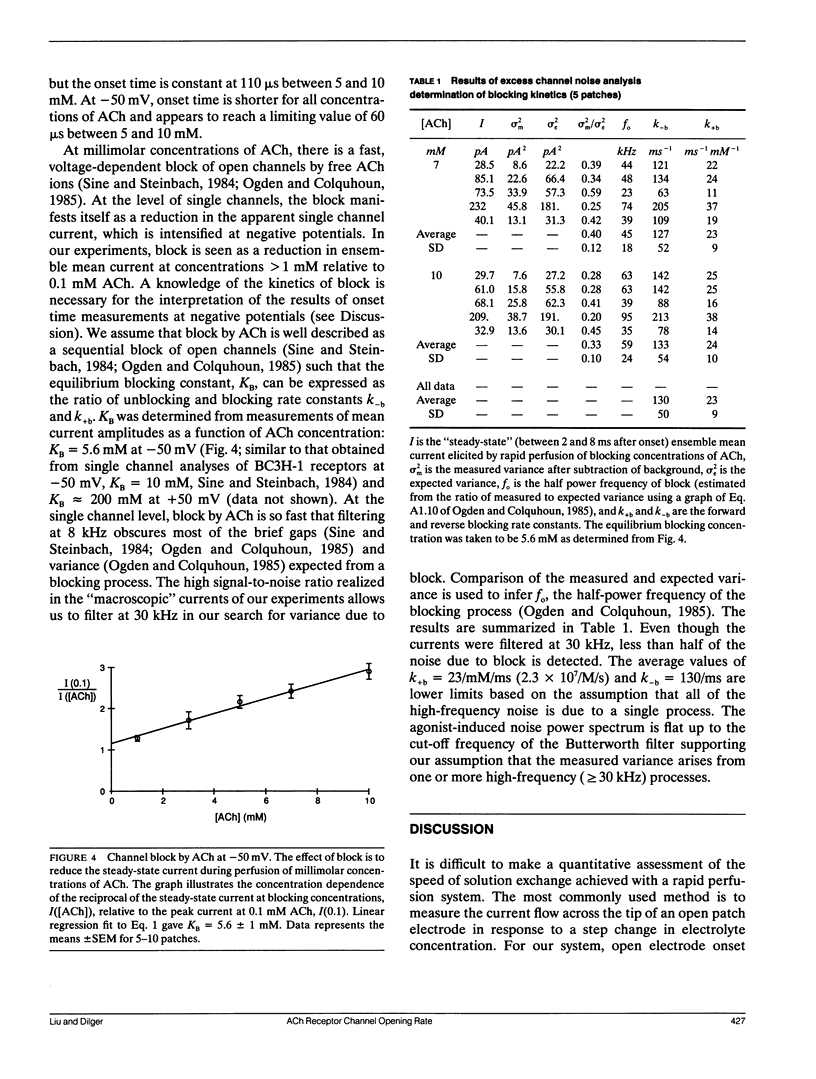
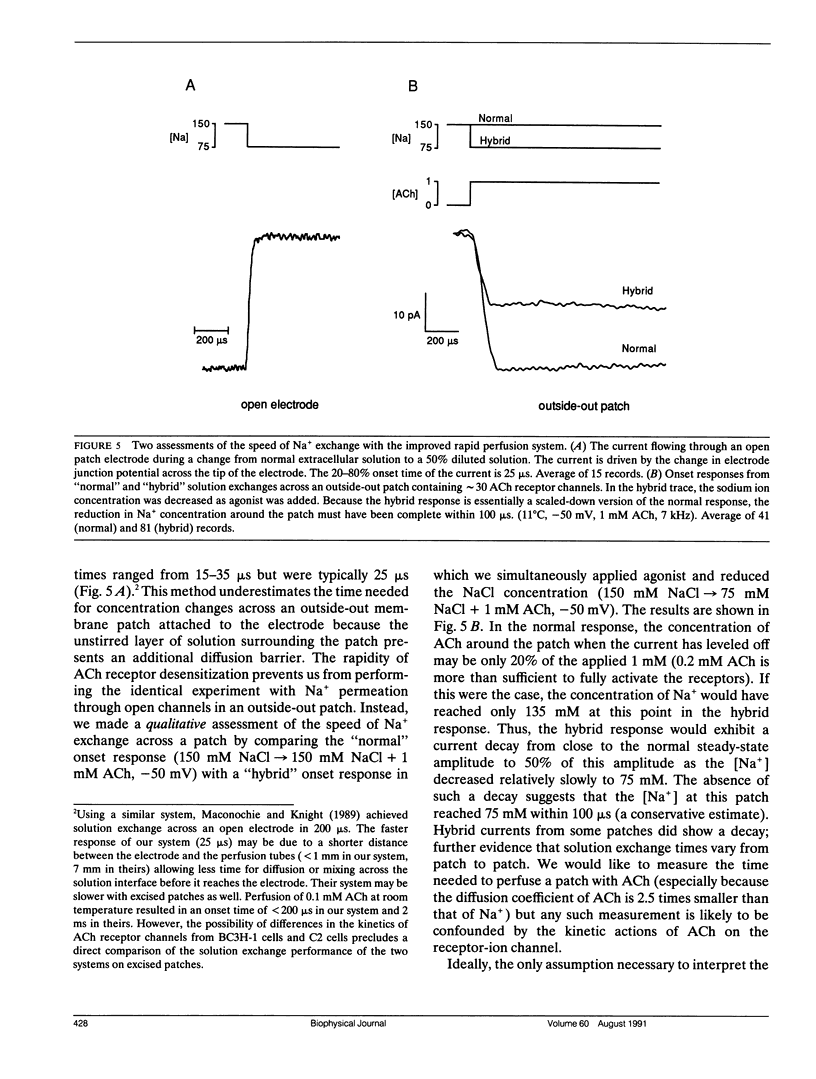
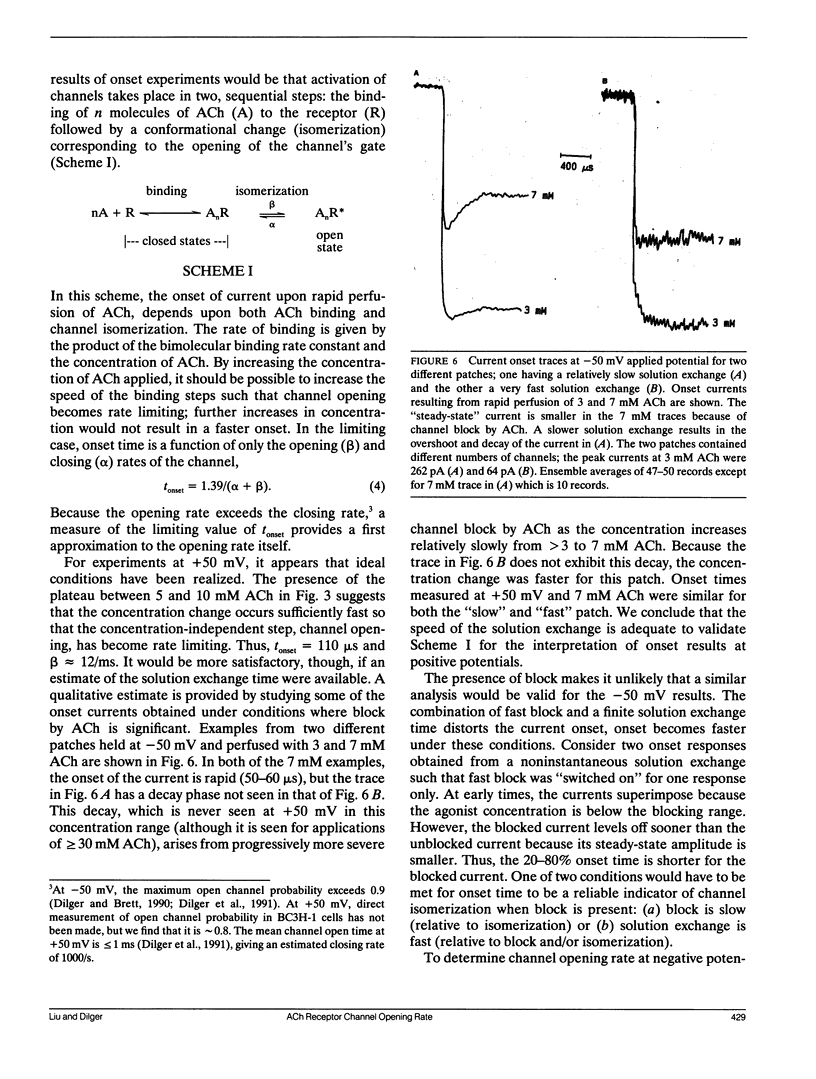
The nicotinic acetylcholine (ACh) receptor is responsible for rapid conversion of chemical signals to electrical signals at the neuromuscular junction. Because the receptor and its ion channel are components of a single transmembrane protein, the time between ACh binding and channel opening can be minimized. To determine just how quickly the channel opens, we made rapid (100-400 microseconds) applications of 0.1-10 mM ACh to outside-out, multichannel membrane patches from BC3H-1 cells, while measuring the onset of current flow through the channels at 11 degrees C. Onset time is steeply dependent upon ACh concentration when channel activation is limited by binding of ACh (0.1-1 mM). At +50 mV, the 20-80% onset time reaches a plateau near 110 microseconds above 5 mM ACh as channel opening becomes rate limiting. Thus, we calculate the opening rate, beta = 12/ms, without reference to specific channel activation schemes. At -50 mV, the combination of a rapid, voltage-dependent block of channels by ACh with a finite solution exchange time distorts onset. To determine opening rate at -50 mV, we determine the kinetic parameters of block from "steady-state" current and noise analyses, assume a sequential model of channel activation/block, and numerically simulate current responses to rapid perfusion of ACh. Using this approach, we find beta = 15/ms. In contrast to the channel closing rate, the opening rate is relatively insensitive to voltage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990 Apr;57(4):723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S., Poppers D. M., Liu Y. The temperature dependence of some kinetic and conductance properties of acetylcholine receptor channels. Biochim Biophys Acta. 1991 Apr 2;1063(2):253–258. doi: 10.1016/0005-2736(91)90379-m. [DOI] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M. The rising phase of the miniature endplate current at the frog neuromuscular junction. Biochim Biophys Acta. 1981 Aug 6;646(1):51–60. doi: 10.1016/0005-2736(81)90271-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Harris W. V., Salpeter E. E., Salpeter M. M. Diffusion and binding constants for acetylcholine derived from the falling phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1594–1598. doi: 10.1073/pnas.81.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Acetylcholine receptor site density affects the rising phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3736–3740. doi: 10.1073/pnas.77.6.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconochie D. J., Knight D. E. A method for making solution changes in the sub-millisecond range at the tip of a patch pipette. Pflugers Arch. 1989 Sep;414(5):589–596. doi: 10.1007/BF00580996. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


