Abstract
The misuse of antibiotics has selected for bacteria that have evolved mechanisms for evading the effects of these drugs. For aminoglycosides, a group of clinically important bactericidal antibiotics that target the A-site of the 16S ribosomal RNA, the most common mode of resistance is enzyme-catalyzed chemical modification of the drug. While aminoglycosides are structurally diverse, a single enzyme can confer resistance to many of these antibiotics. For example, the aminoglycoside kinase APH(3′)-IIIa, produced by pathogenic Gram-positive bacteria such as enterococci and staphylococci, is capable of detoxifying at least 10 distinct aminoglycosides. Here we describe the crystal structures of APH(3′)-IIIa in complex with ADP and kanamycin A or neomycin B. These structures reveal that the basis for this enzyme’s substrate promiscuity is the presence of two alternative subsites in the antibiotic binding pocket. Furthermore, comparison between the A-site of the bacterial ribosome and APH(3′)-IIIa shows that mimicry is the second major factor in dictating the substrate spectrum of APH(3′)-IIIa. These results suggest a potential strategy for drug design aimed at circumventing antibiotic resistance.
Keywords: antibiotic resistance/crystal structure/functional mimicry/kinase/multidrug binding
Introduction
The introduction of antibiotics in the 1940s was thought to have eliminated the scourge of all infectious diseases. However, due to the widespread use and misuse of antibiotics, bacterial resistance to antibiotics has become a serious public health problem. Some of these resistant strains, such as vancomycin-resistant enterococci (VRE) and multidrug resistant Staphylococcus aureus (MRSA), are capable of surviving the effects of most, if not all, antibiotics currently in use (Schentag et al., 1998; Cohen, 2000; Walsh, 2000; Nicolaou and Boddy, 2001). Resistant bacteria can counter the effects of antibiotics in several ways; for aminoglycosides, a group of clinically important bactericidal antibiotics including gentamicin and amikacin, the most common mode of resistance is enzyme-catalyzed chemical modification of the drug (Wright et al., 1998; Mingeot-Leclercq et al., 1999; Kotra et al., 2000). Ordinarily, an aminoglycoside exerts its bactericidal effect by binding to the A-site of the 16S ribosomal RNA, inducing conformational changes in key nucleotides in the decoding center, thus affecting the fidelity of protein translation (Moazed and Noller, 1987; Fourmy et al., 1996; Carter et al., 2000; Pape et al., 2000). However, the chemically modified aminoglycoside, through the covalent addition of either a phosphate, an adenylyl or an acetyl group, has reduced affinity for its cellular target and therefore loses its deleterious effects (Shaw et al., 1993).
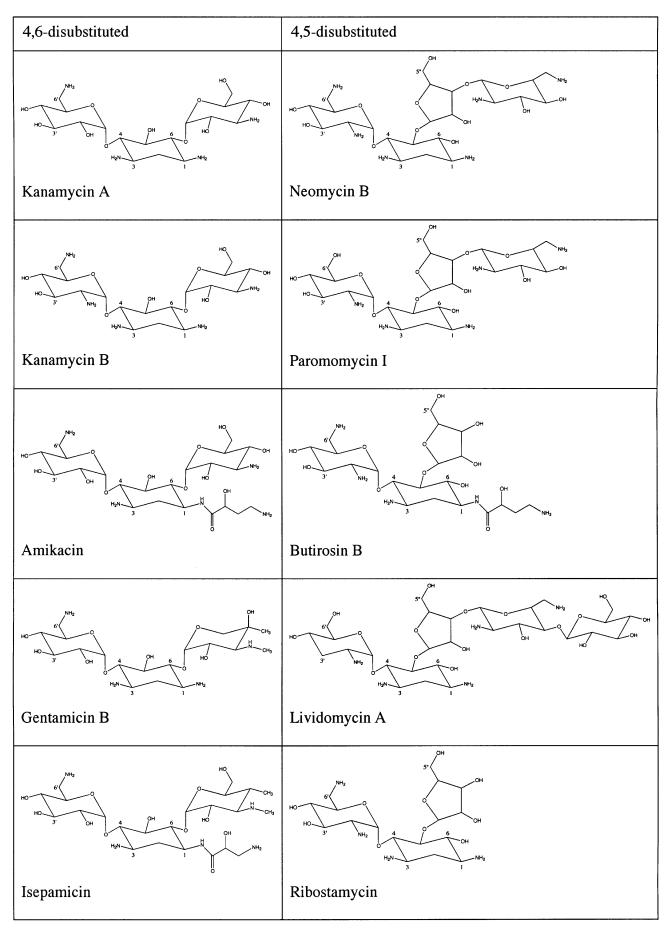
Although structurally diverse, the vast majority of clinically used aminoglycoside antibiotics incorporate either a 4,6- or a 4,5-disubstituted 2-deoxystreptamine ring (Mingeot-Leclercq et al., 1999). Bacterial resistance to multiple members from these two groups can be conferred by the aminoglycoside kinase (3′) type IIIa [APH(3′)-IIIa], an enzyme that catalyzes the ATP-dependent phosphorylation of hydroxyl groups at the 3′ position and/or the 5′′ position (Table I; Wright and Thompson, 1999). Due to the unusually broad spectrum of aminoglycosides that can be detoxified by APH(3′)-IIIa (McKay et al., 1994; Wright and Thompson, 1999), much effort has been put into understanding the structural basis for its promiscuity in substrate recognition. For example: the crystal structures of APH(3′)-IIIa in apo, ADP- and 5′-adenylylimidodiphosphate (AMP-PNP)-bound forms have been determined (Hon et al., 1997; Burk et al., 2001); conformations of several aminoglycosides, such as amikacin and butirosin A, bound to APH(3′)-IIIa, have been studied using nuclear magnetic resonance (NMR; Cox et al., 1996, 2000; Cox and Serpersu, 1997; Mohler et al., 1997); the binding of aminoglycosides to APH(3′)-IIIa has been explored by examining the binding properties of synthetically constructed aminoglycoside variants (McKay et al., 1996); and site-directed mutagenesis studies in combination with molecular docking experiments have been performed in order to predict the arrangement and conformation of different aminoglycosides in APH(3′)-IIIa (Thompson et al., 1999). These studies indicate the importance of electrostatic interactions in enzyme–substrate recognition, and suggest that different aminoglycosides may have radically different conformations in the active site. However, no consensus can be established on the binding mode of aminoglycosides to APH(3′)-IIIa, and thus, the structural basis for broad substrate specificity has remained enigmatic.
Table I. Structures of APH(3′)-IIIa substrates.
The enzyme modifies all 4,6-disubstituted aminoglycosides at the 3′-hydroxyl. All 4,5-disubstituted aminoglycosides, except lividomycin A, are modified at the 3′- and 5′′-hydroxyl groups. Lividomycin A has no hydroxyl group at the 3′ site, hence it can only be phosphorylated at the 5′′ position.
Here we describe the three-dimensional (3D) crystal structure of APH(3′)-IIIa in complex with ADP and either the 4,6-disubstituted aminoglycoside kanamycin A or the 4,5-disubstituted aminoglycoside neomycin B. These structures reveal the binding modes of the aminoglycosides and also how the diverse structures of the substrates are accommodated by a versatile binding site consisting of three subsites. Although the overall structure of APH(3′)-IIIa is distinct from the ribosome, the arrangement of the amino acid side-chains in the binding site of APH(3′)-IIIa imitates that of the nucleotides in the A-site of the ribosome. These results have led us to suggest possible strategies for the design of novel antibacterial treatments.
Results
Overall structure of aminoglycoside-bound APH(3′)-IIIa
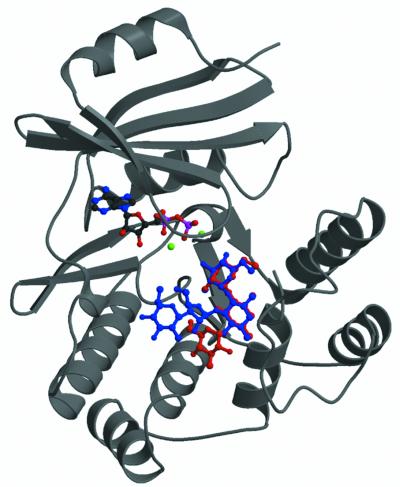
The crystal structures of APH(3′)-IIIa in complex with ADP and either kanamycin A or neomycin B were solved by molecular replacement using the previously determined ADP complex as a search model (Hon et al., 1997) (Figure 1). The structure of the enzyme in complex with ADP and kanamycin A has been refined to 2.4 Å with an R-factor of 0.234 and an R-free of 0.291 (Figure 2A). The APH(3′)-IIIa–ADP–neomycin B complex has been refined to 2.7 Å, and a final R-factor and R-free of 0.230 and 0.308 (Figure 2B), respectively. These two crystal structures represent the first aminoglycoside kinase structures with a bound antibiotic substrate. Crystal structures are now available for APH(3′)-IIIa in the apo-form (Burk et al., 2001), the holo-form (i.e. with bound nucleotides ADP and the ATP analogue AMP-PNP) (Hon et al., 1997; Burk et al., 2001) and the ternary complex (i.e. with bound ADP and kanamycin A or neomycin B). Comparison between the four crystal structures of APH(3′)-IIIa shows that most of the APH(3′)-IIIa protein structure appears to be rather rigid, and no gross domain movements are observed. Four segments can be identified that display differing conformations in the various APH(3′)-IIIa structures: residues 21–26, 100–112, 147–170 and 226–238. Conformational differences observed for residues 100–112 and 226–238 can be attributed to the inherent flexibility present in these segments, as they are invariably associated with high thermal factors and poor electron density in 2Fo–Fc and omit maps.
Fig. 1. Crystal structures of APH(3′)-IIIa in complex with ADP and kanamycin A or neomycin B. Ribbon representation of the APH(3′)-IIIa ternary complexes showing the location of the antibiotic binding site. Kanamycin (red) and neomycin (blue) are superimposed in the binding site. Magnesium ions are shown in green. Since the protein structure does not differ significantly between the two ternary complexes, only one is shown.
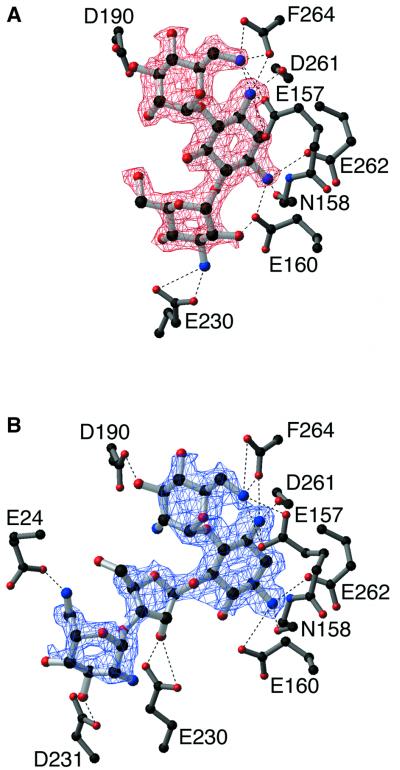
Fig. 2. (A) Simulated annealing Fo–Fc omit map for kanamycin A (contoured at 2σ). Residues forming hydrogen bond interactions are shown. (B) Simulated annealing Fo–Fc omit map for neomycin B (contoured at 2σ), also showing residues that form hydrogen bond interactions with this antibiotic.
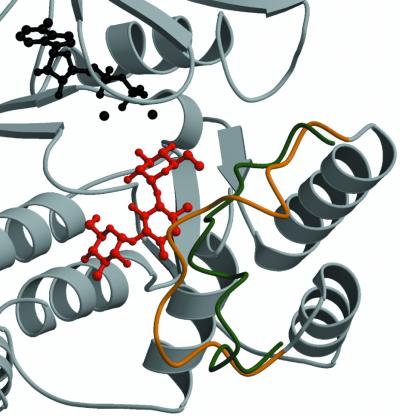
Conformational differences in residues 21–26 and 147–170 observed between the apo-form, the holo-form and the ternary complex structures of aminoglycoside kinase are associated with the nature of bound substrates. The conformation of residues 21–26, which form a loop above the phosphate moieties of the nucleotide and which are structurally homologous to the Gly-X-Gly-X-X-Gly motif in the related protein kinase superfamily, is dependent on the presence or absence of the nucleotide cofactor. A detailed analysis of this has been reported previously (Burk et al., 2001). Residues 147–170 adopt differing conformations depending on the presence or absence of antibiotic substrates. This segment, termed the aminoglycoside-binding loop, is located between two helices (αA and αB). Previous crystallographic studies suggest that in the absence of aminoglycosides this loop is highly flexible (Burk et al., 2001). In the kanamycin A- and neomycin B-bound structures of APH(3′)-IIIa, the aminoglycoside-binding loop is folded over towards the antibiotic, and the shift observed at the tip of the loop (residue 160) is ∼10 Å compared with the holoenzyme crystal structure (Figure 3). As a consequence of the conformational changes in the aminoglycoside-binding loop upon substrate binding, several residues located in this loop are in a position to form interactions with antibiotics, specifically Glu157, Asn158 and Glu160 (Figure 2). In effect, the shift in the aminoglycoside-binding loop results in the completion of the aminoglycoside-binding pocket.
Fig. 3. Structural changes observed in APH(3′)-IIIa upon aminoglycoside binding. Shown is a ribbon diagram of the APH(3′)-IIIa– kanamycin A ternary complex in the vicinity of the antibiotic binding pocket. The Cα trace of the aminoglycoside-binding loop (residues 150–165) is in gold, the remainder of the protein is in gray, the antibiotic is red, and the ADP co-factor and the magnesium ions are displayed in black. Overlaid is a backbone trace for residues 150–165 of the APH(3′)-IIIa–ADP holoenzyme structure (Burk et al., 2001), colored in green. The aminoglycoside-binding loop represents the largest conformational difference between ternary complexes and holoenzyme structures.
Aminoglycoside-binding site
The vast majority of residues located in the aminoglycoside-binding pocket are acidic in nature (three aspartic acids, five glutamic acids and one C-terminal carboxylic acid group), and as a consequence, the pocket is highly negatively charged (Figure 2). In fact, the enzyme provides only acceptor groups for hydrogen bond interactions with aminoglycoside substrates. The abundance of acidic residues in the aminoglycoside-binding pocket can be readily explained by noting that aminoglycosides are invariably positively charged molecules. The presence of negatively charged pockets (putatively) for substrate binding has also been observed in enzymes that either adenylate or acetylate aminoglycosides so as to confer resistance (Perdersen et al., 1995; Wolf et al., 1998; Wybenga-Groot et al., 1999; Kotra et al., 2000). Related to the abundance of acidic residues is the large number of bifurcated hydrogen bonds (Figure 2). This feature of the aminoglycoside-binding pocket may provide for an inherent plasticity, allowing for various aminoglycosides to be bound to APH(3′)-IIIa.
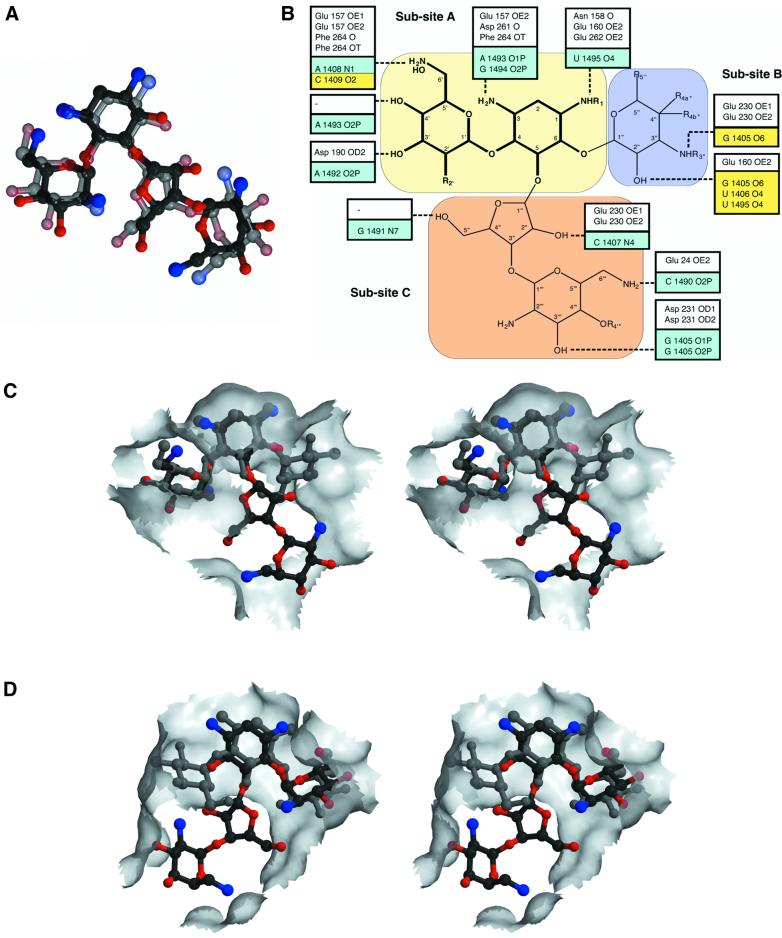
The APH(3′)-IIIa aminoglycoside-binding pocket in both the kanamycin A- and neomycin B-bound ternary complexes can be considered as consisting of three distinct subsites. Subsite A forms interactions with the 2-deoxystreptamine ring, and the hexose substituted at position 4 (often referred to as the prime ring). Although the functional groups may vary, these two rings are the common moieties among most aminoglycosides (Mingeot-Leclercq et al., 1999), and have been shown to be the minimum essential components required for antibacterial activity (Fourmy et al., 1998; Kotra et al., 2000). Subsite B can form interactions with moieties located at the 6 position of the 2-deoxystreptamine ring, e.g. the so-called double-prime ring of kanamycin A. Since only a subset of aminoglycoside substrates of APH(3′)-IIIa have 6-substituted 2-deoxystreptamine rings, subsite B is not always employed. Those aminoglycosides that are substituted at the 5 instead of the 6 position (e.g. neomycin B) employ the alternative subsite C for binding to APH(3′)-IIIa.
Most of the hydrogen bond interactions between the enzyme and kanamycin A or neomycin B are seen to occur in the A subsite. Of specific interest are the interactions between the C-terminal carboxylic acid group and the 2-deoxystreptamine and prime rings, as well as the interaction between Asp190 and the 3′ OH group. The involvement of the C-terminus in substrate binding was previously predicted (Thompson et al., 1999) and the Asp190–3′OH hydrogen bond is significant in that the aminoglycoside 3′hydroxyl group is the site of phosphorylation by APH(3′)-IIIa; also, Asp190 has been suggested to be the catalytic base in the reaction mechanism (Hon et al., 1997). When comparing kanamycin A and neomycin B binding to subsite A, it is intriguing to note that the two aminoglycosides have different functional groups at the 2′ position (OH and NH2 for kanamycin A and neomycin B, respectively) and that the enzyme does not interact with this variable substituent. Furthermore, no hydrogen bond interactions are observed with the 5-hydroxyl group of the 4,6-disubstituted 2-deoxystreptamine ring of kanamycin A, or with the 6-hydroxyl group of the 4,5-disubstituted 2-deoxystreptamine ring of neomycin B.
As indicated above, both subsites B and C provide many fewer hydrogen bond interactions with the aminoglycoside antibiotic than subsite A. This limited number of specific interactions mirrors the greater variability present in the components that can occupy these two subsites, and hydrogen bonds are only made with functional groups that are highly conserved within the 4,6- or 4,5-disubstituted 2-deoxystreptamine aminoglycosides (Table I). Relating to this is the fact that subsite B is much smaller in size than subsite C. While subsite B has to provide room for only one hexose ring, subsite C may be occupied by one (ribostamycin, butirosin B), two (neomycin B, paromomycin I) or three (lividomycin A) rings.
Discussion
Substrate-binding mechanism of other multiple substrate-recognizing enzymes
The ternary complexes of APH(3′)-IIIa reported here are the first aminoglycoside kinase structures to be solved with a bound aminoglycoside. To date, only one other aminoglycoside-detoxifying enzyme, ANT(4′)-Ia, has been determined in the presence of an antibiotic substrate, kanamycin A (Perdersen et al., 1995). Similar to APH(3′)-IIIa, ANT(4′)-Ia is capable of binding multiple substrates and its antibiotic-binding site is lined with several glutamate residues, thus providing a negatively charged product. However, a single ternary ANT(4′)-Ia complex has provided little insight into how diverse aminoglycosides bind to a single aminoglycoside-resistance enzyme.
In addition to aminoglycoside antibiotic-resistance enzymes, many other types of proteins are also capable of binding to diverse substrates. A few examples include various multidrug resistance (MDR) transporters, MDR transporter transcription activators and P450s. Extensive research has been carried out in an attempt to elucidate the basis of broad substrate recognition by these enzymes; however it was not until recently, when crystal structures of some of these proteins were determined in the presence of substrates or inhibitors, that multi-substrate recognition could be examined in atomic detail. The results indicate that diverse substrate recognition and binding are mediated in part by flexible domain movement, compliant and rigid sections in the active site, electrostatic interactions, and/or distinct substrate-binding subsites. For example, type II 3-hydroxyacyl-CoA dehydrogenase (HADH II) (Powell et al., 2000) and aromatic amino acid aminotransferase (AroAT) (Okamoto et al., 1998) both contain highly flexible regions that move significantly to close the active site upon substrate binding. Further analysis of AroAT bound to structurally related inhibitors shows that the active site can be divided into regions of rigidity and flexibility (Okamoto et al., 1999). The residues in the rigid region remain in identical conformations upon binding of various inhibitors. These residues interact with the portion of the substrate that is involved in the catalytic reaction, to determine specificity and properly orient the substrate for efficient catalysis. Conversely, the residues in the flexible section of the binding site can adopt different conformations depending on the shape and size of the inhibitor bound. Additionally, compliance with structurally diverse substrates can also be facilitated by multiple and overlapping substrate-binding subsites, as observed in QacR (Schumacher et al., 2001), a MDR transporter regulator. Another strategy employed by QacR, and a number of other MDR transporter regulators that bind cationic substrates such as BmrR (Zheleznova et al., 1999), is the presence of glutamate residues buried in the binding pocket. The carboxylate group of glutamate complements the positive charges on the cationic substrates and properly orients the substrate for catalysis.
These characteristics of substrate recognition and binding are also mirrored in APH(3′)-IIIa. In APH(3′)-IIIa, the flexible antibiotic-binding loop moves into close proximity to, and forms key interactions with the aminoglycoside. The residues that interact with the 2-deoxystreptamine ring and the hexose at the 4 position in both ternary complexes of APH(3′)-IIIa have essentially the same conformations, whereas the residues that interact with the remainder of the substrates are more flexible. The side-chains of these residues (Glu230, Asp231 and Glu24) differ in the kanamycin A and neomycin B complexes. This is further supported by the higher than average thermal factor values of these residues. Another element of aminoglycoside binding to APH(3′)-IIIa is electrostatic interaction (Thompson et al., 1999). Aminoglycosides are cationic molecules and their binding site in APH(3′)-IIIa is a negatively charged groove lined with glutamate and aspartate residues. Furthermore, the active site is composed of three distinct subsites in order to accommodate structurally different components of the substrate.
Comparison of aminoglycoside-binding mode in 16S ribosomal RNA versus APH(3′)-IIIa
As stated above, the intended cellular target for aminoglycosides that can be detoxified by APH(3′)-IIIa is the A-site of the bacterial ribosome (Wright et al., 1998; Mingeot-Leclercq et al., 1999; Kotra et al., 2000). Solution structures of a fragment of the A-site in complex with gentamicin C1a and paromomycin I have been determined (Fourmy et al., 1996; Yoshizawa et al., 1998), and the crystal structure for the entire 30S ribosome in complex with various antibiotics, including paromomycin I, has been determined (Carter et al., 2000). Of these structures, the complex structures with paromomycin I are of particular interest here, since this aminoglycoside is also a substrate for APH(3′)-IIIa. Paromomycin I is a 4,5-disubstituted aminoglycoside that differs from neomycin B in one functional group at position 6, where it possesses a hydroxyl instead of an amino group (Table I). Comparison between paromomycin I and neomycin B bound to the 16S ribosomal RNA (the intended target) and APH(3′)-IIIa (a decoy for the antibiotics), respectively, can provide an insight into the basis of the effectiveness of antibiotic-resistance mechanisms.
Results from a comparison between neomycin B bound to APH(3′)-IIIa versus paromomycin I bound to the ribosome can be summarized in three main points. First, the conformations of neomycin B and paromomycin I are effectively identical [root mean square deviation (r.m.s.d.) 1.7 Å; Figure 4A]. This observation is surprising, considering the large number of conformations these antibiotics have been shown to exhibit (Mikkelsen et al., 2001). A probable explanation for this is that enzymes involved in the biosynthesis of aminoglycosides have evolved to produce products which, in their lowest energy conformation, are optimal for binding the A-site of the bacterial ribosome, so as to enhance binding affinity through curtailing loss of entropy. Aminoglycoside modifying enzymes such as APH(3′)-IIIa in turn evolved to capture this lowest-energy conformer, so as to effectively compete with the ribosome. Support for this explanation comes from energy calculations (data not shown), which confirm that the observed conformations of the two aminoglycosides correspond to their minimum-energy conformers. Secondly, the functional groups of the two aminoglycosides that are utilized in binding to APH(3′)-IIIa or to the bacterial ribosome are identical, with the exception of two, which are not employed for binding in the antibiotic-resistance enzyme (Figure 4B). As expected, these functional groups correspond to those moieties that are predominantly conserved among 4,5-disubstituted aminoglycosides. Thirdly, while the conformation of the aminoglycosides and the functional groups utilized for binding are effectively identical when comparing the neomycin B-bound structure of APH(3′)-IIIa and the paromomycin I-bound structure of the 30S ribosome, there are significant differences when examining the van der Waals interactions. The most striking difference is that the face of the aminoglycoside that forms most of the van der Waals interactions with APH(3′)-IIIa is opposite to that which interacts with the 16S ribosomal RNA (Figure 4C and D).
Fig. 4. Comparison of aminoglycoside binding to APH(3′)-IIIa versus the bacterial ribosome. (A) Superposition of neomycin B, in the conformation observed in the APH(3′)-IIIa ternary complex, and paromomycin I in the conformation observed in the crystal structure of the 30S ribosomal subunit (Carter et al., 2000). Neomycin B is shown in solid colors and paromomycin I is semi-transparent. (B) Schematic overview of hydrogen bond interactions made by aminoglycosides with APH(3′)-IIIa and the bacterial ribosome (Fourmy et al., 1998; Yoshizawa et al., 1998; Carter et al., 2000). A combined generic chemical structure for 4,6- and 4,5-disubstituted aminoglycosides is shown, highlighting common functional groups (see also Table I). Hydrogen bond interactions made by APH(3′)-IIIa with aminoglycosides are shown in white boxes, while those made by the ribosome, as observed in the ribosome–paromomycin I crystal structure (Carter et al., 2000), are shown in aqua. Additional interactions made by the ribosome, as observed in NMR studies of neomycin B and gentamycin C1a (Fourmy et al., 1998; Yoshizawa et al., 1998), are displayed in yellow boxes. (C) Stereo view of the van der Waals surface of the APH(3′)-IIIa aminoglycoside-binding pocket. Also shown are kanamycin A and neomycin B. (D) Stereo view of the van der Waals surface of the bacterial ribosomal aminoglycoside-binding pocket. Also shown are paromomycin I and a modeled kanamycin A. This stereo view has been subjected to a 180° rotation around the vertical axis with respect to (C), to show that opposite faces of the aminoglycosides form predominant van der Waals interactions with either the ribosome or APH(3′)-IIIa.
The results of the analysis of 4,5-disubstituted aminoglycoside binding to the ribosome and to APH(3′)-IIIa, i.e. identical conformation of antibiotic and nearly identical hydrogen bond interactions, but differing van der Waals interactions, is likely to also extend to 4,6-disubstituted aminoglycosides such as kanamycin A. Modeling of kanamycin A into the ribosome A-site, employing the conformation seen in the APH(3′)-IIIa ternary complex and employing hydrogen bond interactions akin to those observed with paromomycin I, results in a structure that is completely consistent with structural and functional studies of gentamicin C1a binding to the ribosome (Figure 4B and D) (Yoshizawa et al., 1998).
The above analyses provide an explanation for the structural basis of substrate promiscuity of APH(3′)-IIIa and its effectiveness as an aminoglycoside antibiotic-resistance enzyme. The aminoglycoside-binding pocket in APH(3′)-IIIa mimics in nearly every important aspect the intended target site for these antibiotics, namely the A-site of the prokaryotic ribosome.
Although RNA and protein are chemically very different and are not expected to form the same shapes or to feature the same chemical properties, macromolecular mimicry between RNAs and proteins is not uncommon. The foremost example of mimicry is of Phe-tRNA-EF-Tu (Nissen et al., 1995) by EF-G (Czworkowski et al., 1994; al-Karadaghi et al., 1996). The overall structure of the two complexes is highly similar, and the shape of part of the EF-G resembles the anticodon stem of the tRNA (Liljas, 1996; Nissen et al., 2000). In addition to structural resemblance, molecular mimicry can also be defined based on common functions (Keene, 1996). For example, a specific RNA sequence can act as a decoy for antibodies specific for an autoantigenic epitope of the human insulin receptor (Doudna et al., 1995). In our case, APH(3′)-IIIa acts as a decoy binding site for antibiotics that target the 16S ribosomal RNA. Although they do not resemble each other in the shape of the overall structure, APH(3′)-IIIa and the 16S ribosome utilize a highly homologous hydrogen bonding scheme for binding the same spectrum of substrates. In contrast to the other examples of molecular mimicry, where the protein and RNA during their biological functions, may bind to another protein or RNA, the situation observed here is unique in that the protein and RNA interact with a small molecule instead.
While mimicry of the ribosomal A-site by APH(3′)-IIIa provides a structural explanation for this enzyme’s effectiveness as a resistance factor, it also raises concerns with respect to the development of new antibiotics that target the 16S RNA. However, the observation that APH(3′)-IIIa and the ribosome differ in one crucial aspect with respect to aminoglycoside binding, namely in van der Waals interactions, suggests that there may exist possible strategies for the design of novel variant aminoglycoside antibiotics that can interact with the ribosome A-site but are unable to be detoxified by APH(3′)-IIIa and related enzymes.
Materials and methods
Crystallization and data collection
APH(3′)-IIIa was obtained using previously established procedures (McKay et al., 1994). Crystals of ternary complexes were grown at 4°C using the hanging drop vapor diffusion method by combining 2 µl of a solution containing 12–15 mg/ml protein, 2.5 mM ADP and 2.5 mM aminoglycoside antibiotic with 2 µl of 35–40% (v/v) polyethylene glycol 600 and 0.1 M 2-(N-cyclohexylamino)ethane sulfonic acid (CHES) pH 9.0–9.5, and equilibrating against 0.7 ml of the same solution. Crystals reached a maximal size of 0.25 × 0.25 × 0.1 mm in ∼4 weeks. These crystals belonged to the tetragonal space group P4322, with unit cell dimensions a = b = 46.6 Å, c = 301 Å. All data collection was performed under cryogenic conditions (110 K). Prior to data collection, crystals were soaked for ∼1 min in 0.1 M CHES pH 9.0–9.5 and 55% (v/v) polyethylene glycol 600, and flash frozen in liquid nitrogen. Diffraction data for APH(3′)-IIIa–ADP–kanamycin A were collected from two crystals; data from the first crystal were collected on a Rigaku rotating copper anode X-ray generator using a Mar imaging plate to 2.9 Å, and data from a second crystal were collected at the X8C beamline of the National Synchrotron Light Source (Brookhaven National Laboratories) using an Area Detector Systems Corp. (ADSC) Quantum 4 charge-coupled device (CCD) detector (λ = 1.072 Å) to 2.4 Å. Diffraction data for the ternary complex containing neomycin B were collected from a single crystal at the X8C beamline of the National Synchrotron Light Source using an ADSC Quantum 4 CCD detector (λ = 1.072 Å) to 2.7 Å. All data were processed using the HKL suite of programs (Otwinowski and Minor, 1997), resulting in the statistics shown in Table II.
Table II. Diffraction data and refinement statistics.
| APH(3′)-IIIa–ADP–kanamycin A | APH(3′)-IIIa–ADP–neomycin B | |
|---|---|---|
| Data collection | ||
| Resolution limit (Å) | 2.4 | 2.7 |
| Data redundancy (outer shell) | 7.4 (2.3) | 3.4 (1.9) |
| Completeness (%) (outer shell) | 88.4 (61.1) | 80.8 (48.2) |
| Rsym/merge (%) (outer shell) | 10.1 (39.9) | 4.8 (16.8) |
| Mean I/σ (I) (outer shell) | 8.0 (1.9) | 11.3 (4.3) |
| Model refinement | ||
| Number of atoms | 2297 | 2285 |
| Rcryst/Rfree | 0.234/0.291 | 0.230/0.308 |
| R.m.s.d. | ||
| Bonds (Å) | 0.007 | 0.008 |
| Angles (°) | 1.321 | 1.393 |
Ten percent of randomly selected reflections were designated as test reflections for use in R cross-validation.
Structure determination and refinement
The structure of APH(3′)-IIIa bound with ADP and kanamycin A was solved by molecular replacement in the crystallography and NMR system (CNS; Brünger et al., 1998), using the APH(3′)-IIIa–ADP complex (Burk et al., 2001) as the search model. After positioning the model in the unit cell, and several cycles of refinement using CNS, the kanamycin A moiety was added adjacent to the nucleotide, based on difference electron density maps (2Fo–Fc and Fo–Fc). Ideal stereochemistry applied to the aminoglycoside during subsequent refinement was based on the crystal structure of kanamycin A (Koyama and Iitaka, 1968). Examination of initial electron density maps also showed that the loop located between helices αA and αB (residues 150–165) required remodeling. Based on difference electron density maps as well as a simulated annealing omit map, this section of the protein structure was rebuilt using the program O (Jones et al., 1991). Successive cycles of refinement were alternated with manual intervention, and the addition of solvent molecules was continued until no significant improvement in model statistics was observed. A partially refined structure of APH(3′)-IIIa–ADP–kanamycin A complex, omitting the antibiotic and solvent molecules, was used to solve the structure of APH(3′)-IIIa bound with ADP and neomycin B. Following rigid body refinement and simulated annealing, the neomycin B moiety was modeled into the positive electron density flanking the nucleotide. Stereochemical restraints employed for refinement of neomycin B were based on those of kanamycin A and information provided by Dr J.R.Cox (Murray State University, KY). Refinement followed a strategy analogous to that described above for the kanamycin A ternary complex of APH(3′)-IIIa. Final refinement statistics for both ternary complex structures are given in Table II.
Coordinates
Coordinates have been deposited in the Protein Data Bank under codes 1L8T and 1L8U for APH(3′)-IIIa in complex with ADP and kanamycin A, and APH(3′)-IIIa in complex with ADP and neomycin B, respectively.
Acknowledgments
Acknowledgements
We thank Dr M.E.Fraser (University of Western Ontario) for her assistance with the collection of APH(3′)-IIIa–ADP–kanamycin A data and Dr J.Cechetto (McMaster University, Ontario) for assistance with energy calculations. This research was supported by a grant from the Canadian Institutes of Health Research. A.M.B. is the recipient of a CIHR/PMAC-HRF Research Career Award and the Premier’s Research Excellence Award.
References
- al-Karadaghi S., Aevarsson,A., Garber,M., Zheltonosova,J. and Liljas,A. (1996) The structure of elongation factor G in complex with GDP: conformational flexibility and nucleotide exchange. Structure, 4, 555–565. [DOI] [PubMed] [Google Scholar]
- Brünger A. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta. Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Burk D.L., Hon,W.C., Leung,A.K.-W. and Berghuis,A.M. (2001) Structural analyses of nucleotide binding to an aminoglycoside phosphotransferase. Biochemistry, 40, 8756–8764. [DOI] [PubMed] [Google Scholar]
- Carter A.P., Clemons,W.M., Brodersen,D.E., Morgan-Warren,R.J., Wimberly,B.T. and Ramakrishnan,V. (2000) Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature, 407, 340–348. [DOI] [PubMed] [Google Scholar]
- Cohen M.L. (2000) Changing patterns of infectious disease. Nature, 406, 762–767. [DOI] [PubMed] [Google Scholar]
- Cox J.R. and Serpersu,E.H. (1997) Biologically important conformations of aminoglycoside antibiotics bound to an aminoglycoside 3′-phosphotransferase as determined by transferred nuclear Overhauser effect spectroscopy. Biochemistry, 36, 2353–2359. [DOI] [PubMed] [Google Scholar]
- Cox J.R., McKay,G.A., Wright,G.D. and Serpesu,E.H. (1996) Arrangement of substrates at the active site of an aminoglycoside antibiotic 3′-phosphotransferase as determined by NMR. J. Am. Chem. Soc., 118, 1295–1301. [Google Scholar]
- Cox J.R., Ekman,D.R., DiGiammarino,E.L., Akal-Strader,A. and Serpersu,E.H. (2000) Aminoglycoside antibiotics bound to aminoglycoside-detoxifying enzymes and RNA adopt similar conformations. Cell Biochem. Biophys., 33, 297–308. [DOI] [PubMed] [Google Scholar]
- Czworkowski J., Wang,J., Steitz,T. and Moore,P. (1994) The crystal structure of elongation factor G complexed with GDP at 2.7 Å resolution. EMBO J., 13, 3661–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna J., Cech,T. and Sullenger,B. (1995) Selection of an RNA molecule that mimics a major autoantigenic epitope of human insulin receptor. Proc. Natl Acad. Sci. USA, 92, 2355–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourmy D., Recht,M.I., Blanchard,S.C. and Puglisi,J.D. (1996) Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science, 274, 1367–1371. [DOI] [PubMed] [Google Scholar]
- Fourmy D., Recht,M.I. and Puglisi,J.D. (1998) Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J. Mol. Biol., 277, 347–362. [DOI] [PubMed] [Google Scholar]
- Hon W.C., McKay,G.A., Thompson,P.R., Sweet,R.M., Yang,D.S., Wright,G.D. and Berghuis,A.M. (1997) Structure of an enzyme required for aminoglycoside antibiotic resistance reveals homology to eukaryotic protein kinases. Cell, 89, 887–895. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.-Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Keene J.D. (1996) RNA surfaces as functional mimetics of proteins. Chem. Biol., 3, 505–513. [DOI] [PubMed] [Google Scholar]
- Kotra L.P., Haddad,J. and Mobashery,S. (2000) Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob. Agents Chemother., 44, 3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama G. and Iitaka,Y. (1968) The crystal structure of kanamycin. Tetrahedron Lett., 15, 1875–1879. [DOI] [PubMed] [Google Scholar]
- Liljas A. (1996) Imprinting through molecular mimicry: protein synthesis. Curr. Biol., 6, 247–249. [DOI] [PubMed] [Google Scholar]
- McKay G., Thompson,P. and Wright,G. (1994) Broad spectrum aminoglycoside phosphotransferase type III from Enterococcus: overexpression, purification and substrate specificity. Biochemistry, 33, 6936–6944. [DOI] [PubMed] [Google Scholar]
- McKay G.A., Roestamadji,J., Mobashery,S. and Wright,G.D. (1996) Recognition of aminoglycoside antibiotics by enterococcal– staphylococcal aminoglycoside 3′-phosphotransferase type IIIa: role of substrate amino groups. Antimicrob. Agents Chemother., 40, 2648–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen N.E., Johansson,K., Virtanen,A. and Kirsebom,L.A. (2001) Aminoglycoside binding displaces a divalent metal ion in a tRNA–neomycin B complex. Nature Struct. Biol., 8, 510–514. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq M.P., Glupczynski,Y. and Tulkens,P.M. (1999) Aminoglycosides: activity and resistance. Antimicrob. Agents Chemother., 43, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D. and Noller,H.F. (1987) Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature, 327, 389–394. [DOI] [PubMed] [Google Scholar]
- Mohler M.L., Cox,J.R. and Serpersu,E.H. (1997) Aminoglycoside phosphotransferase (3′)-IIIa [APH(3′)-IIIa]-bound conformation of the aminoglycoside lividomycin A characterized by NMR. Carbohydr. Lett., 3, 17–24. [Google Scholar]
- Nicolaou K.C. and Boddy,C.N.C. (2001) Behind enemy lines. Sci. Am., 284, 54–61. [DOI] [PubMed] [Google Scholar]
- Nissen P., Kjeldgaard,M., Thirup,S., Polekhina,G., Reshetnikova,L., Clark,B. and Nyborg,J. (1995) Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu and a GTP analogue. Science, 270, 1464–1472. [DOI] [PubMed] [Google Scholar]
- Nissen P., Kjeldgaard,M. and Nyborg,J. (2000) Macromolecular mimicry. EMBO J., 19, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A., Nakai,Y., Hayashi,H., Hirotsu,K. and Kagamiyama,H. (1998) Crystal structures of Paracoccus denitrificans aromatic amino acid aminotransferase: a substrate recognition site constructed by rearrangement of hydrogen bond network. J. Mol. Biol., 280, 443–461. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Ishii,S., Hirotsu,K. and Kagamiyama,H. (1999) The active site of Paracoccus denitrificans aromatic amino acid aminotransferase has contrary properties: flexibility and rigidity. Biochemistry, 38, 1176–1184. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. and Minor,W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol., 276, 307–326. [DOI] [PubMed] [Google Scholar]
- Pape T., Wintermeyer,W. and Rodnina,M.V. (2000) Conformational switch in the decoding region of 16S rRNA during aminoacyl-tRNA selection on the ribosome. Nature Struct. Biol., 7, 104–107. [DOI] [PubMed] [Google Scholar]
- Perdersen L.C., Benning,M.M. and Holden,H.M. (1995) Structural investigation of the antibiotic and ATP-binding sites in kanamycin nucleotidyltransferase. Biochemistry, 34, 13305–13311. [DOI] [PubMed] [Google Scholar]
- Powell A.J., Read,J.A., Banfield,M.J., Gunn-Moore,F., Yan,S.D., Lustbader,J., Stern,A.R., Stern,D.M. and Brady,R.L. (2000) Recognition of structurally diverse substrates by type II 3-hydroxyacyl-CoA dehydrogenase (HADH II)/amyloid-β binding alcohol dehydrogenase (ABAD). J. Mol. Biol., 303, 311–327. [DOI] [PubMed] [Google Scholar]
- Schentag J.J., Hyatt,J.M., Carr,J.R., Paladino,J.A., Birmingham,M.C., Zimmer,G.S. and Cumbo,T.J. (1998) Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium and the importance of antibiotic management and infection control. Clin. Infect. Dis., 26, 1204–1214. [DOI] [PubMed] [Google Scholar]
- Schumacher M.A., Miller,M.C., Grkovic,S., Brown,M.H., Skurray,R.A. and Brennan,R.G. (2001) Structural mechanisms of QacR induction and multidrug recognition. Science, 294, 2158–2163. [DOI] [PubMed] [Google Scholar]
- Shaw K.J., Rather,P.N., Hare,R.S. and Miller,G.H. (1993) Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev., 57, 138–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.R., Schwartzenhauer,J., Hughes,D.W., Berghuis,A.M. and Wright,G.D. (1999) The COOH terminus of aminoglycoside phosphotransferase (3′)-IIIa is critical for antibiotic recognition and resistance. J. Biol. Chem., 274, 30697–30706. [DOI] [PubMed] [Google Scholar]
- Walsh C. (2000) Molecular mechanisms that confer antibacterial drug resistance. Nature, 406, 775–781. [DOI] [PubMed] [Google Scholar]
- Wolf E., Vassilev,A., Makino,Y., Sali,A., Nakatani,Y. and Burley,S.K. (1998) Crystal structure of a GCN5-related N-acetyltransferase: Serratia marcescens aminoglycoside 3-N-acetyltransferase. Cell, 94, 439–449. [DOI] [PubMed] [Google Scholar]
- Wright G.D. and Thompson,P.R. (1999) Aminoglycoside phosphotrans ferases: proteins, structure and mechanism. Front. Biosci., 4, D9–D21. [DOI] [PubMed] [Google Scholar]
- Wright G.D., Berghuis,A.M. and Mobashery,S. (1998) Aminoglycoside antibiotics. Structures, functions and resistance. Adv. Exp. Med. Biol., 456, 27–69. [PubMed] [Google Scholar]
- Wybenga-Groot L.E., Draker,K., Wright,G.D. and Berghuis,A.M. (1999) Crystal structure of an aminoglycoside 6′-N-acetyltransferase: defining the GCN5-related N-acetyltransferase superfamily fold. Structure Fold. Des., 7, 497–507. [DOI] [PubMed] [Google Scholar]
- Yoshizawa S., Fourmy,D. and Puglisi,J.D. (1998) Structural origins of gentamicin antibiotic action. EMBO J., 17, 6437–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznova E.E., Markham,P.N., Neyfakh,A.A. and Brennan,R.G. (1999) Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell, 96, 353–362. [DOI] [PubMed] [Google Scholar]