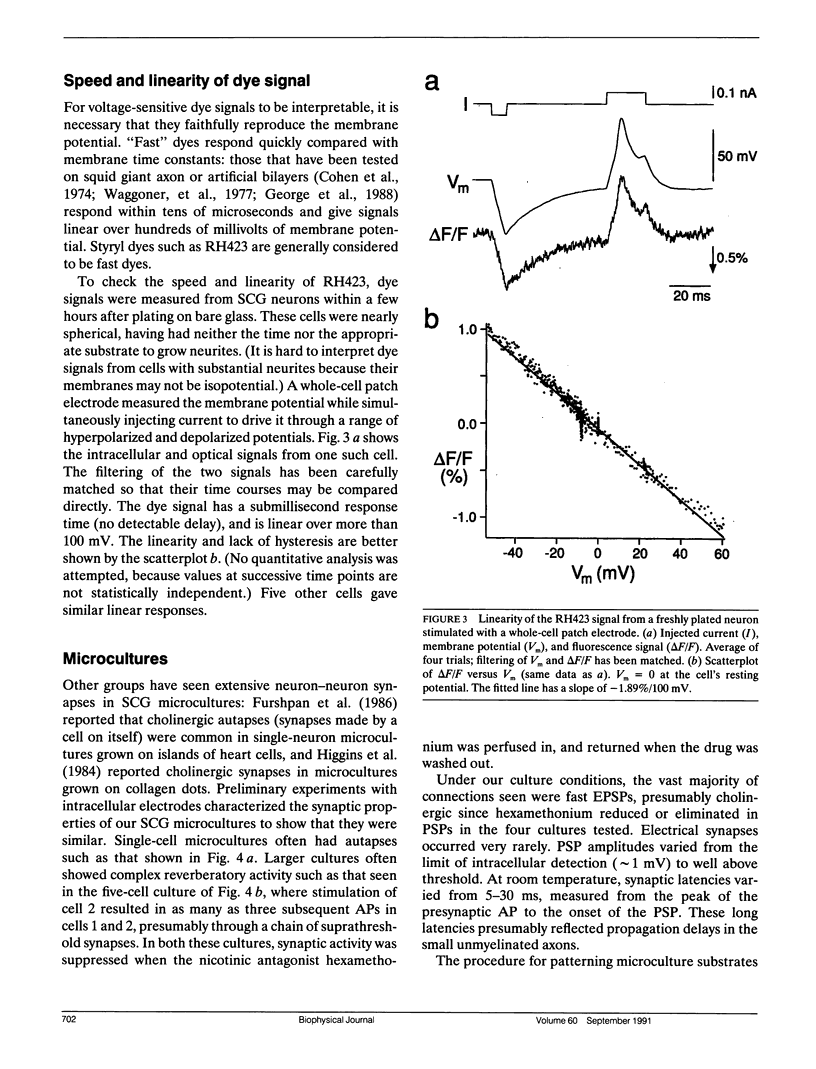
Abstract
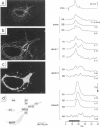
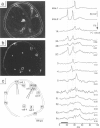
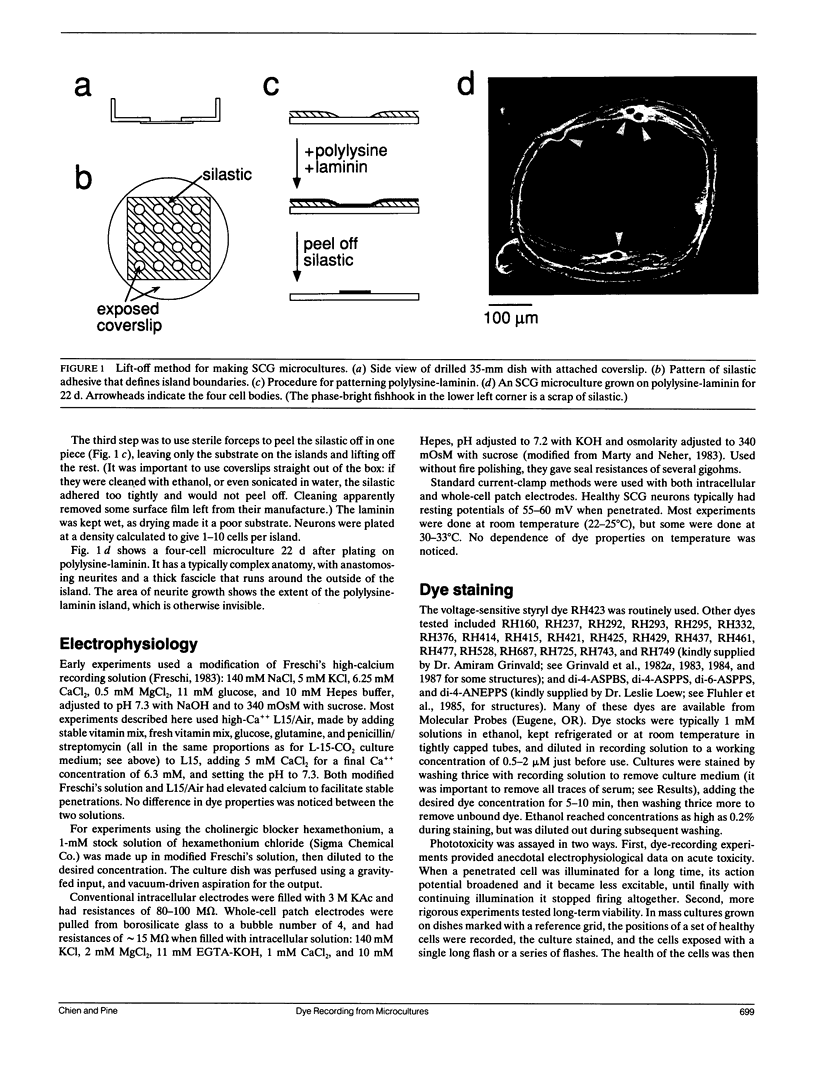
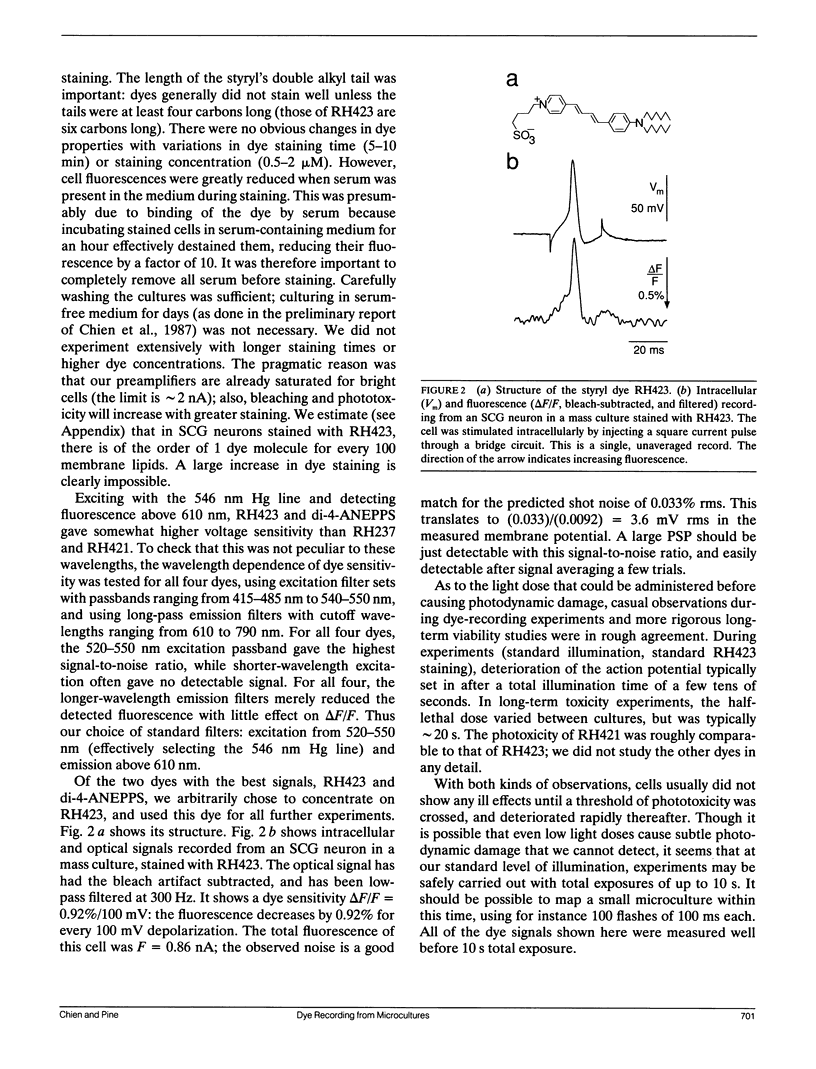
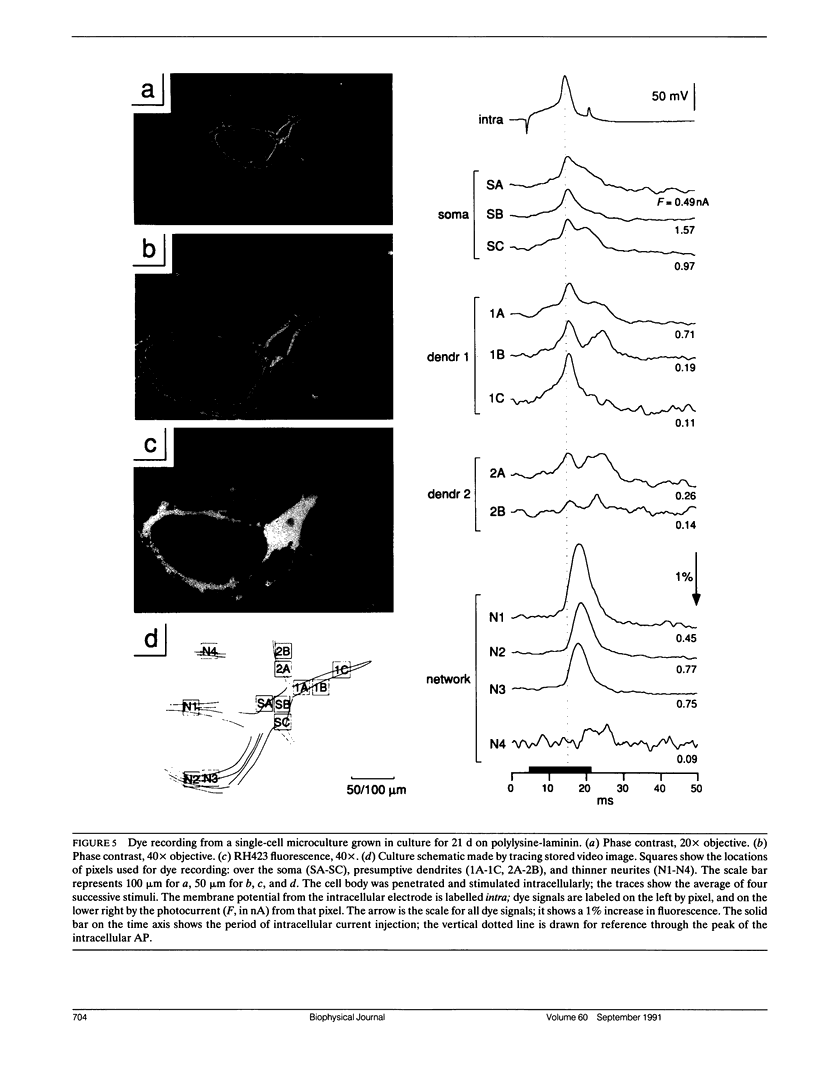
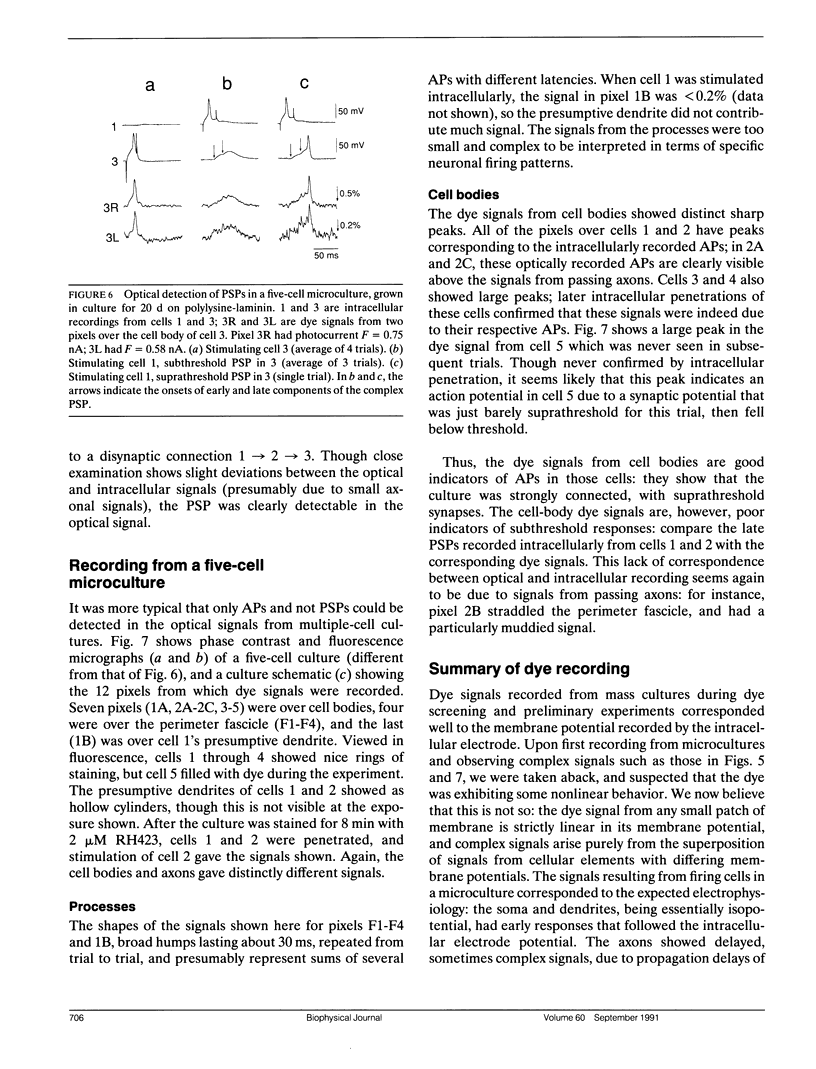
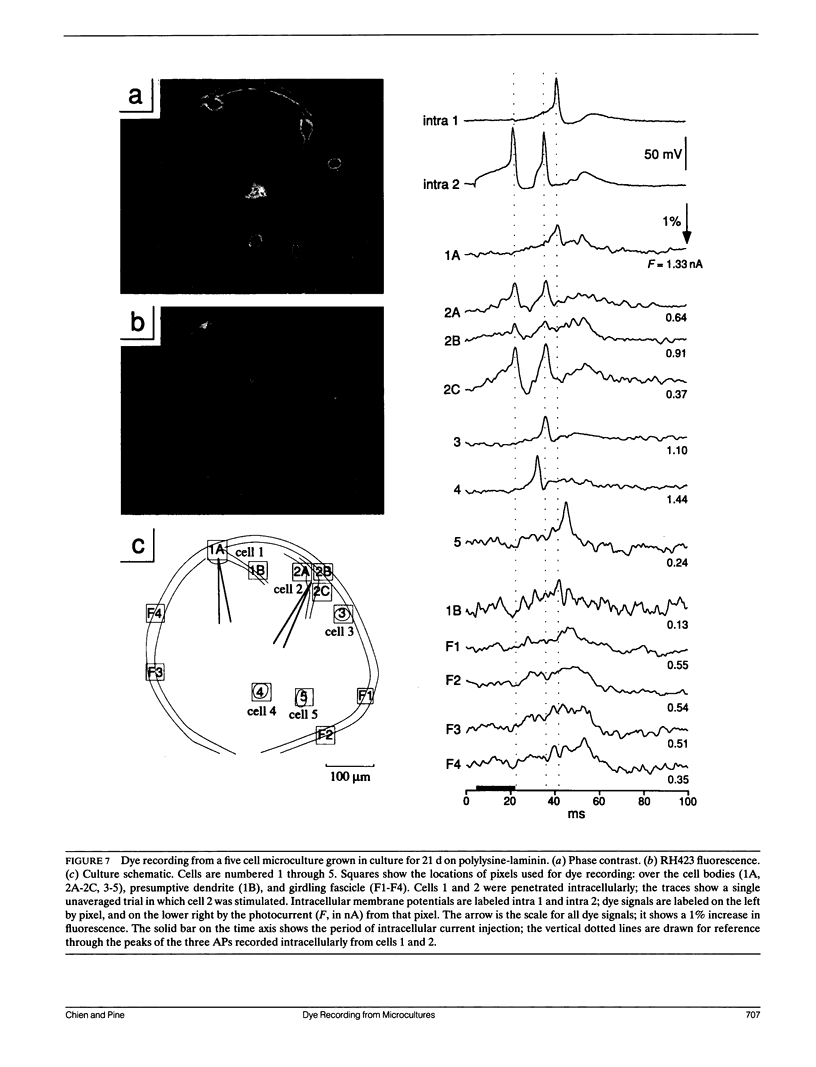
Given the appropriate multicell electrophysiological techniques, small networks of cultured neurons (microcultures) are well suited to long-term studies of synaptic plasticity. To this end, we have developed an apparatus for optical recording from cultured vertebrate neurons using voltage-sensitive fluorescent dyes (Chien, C.-B., and J. Pine. 1991. J. Neurosci. Methods. 38:93-105). We evaluate here the usefulness of this technique for recording action potentials and synaptic potentials in microcultures of neurons from the rat superior cervical ganglion (SCG). After extensive dye screening and optimization of conditions, we chose the styryl dye RH423, which gave fast linear fluorescence changes of approximately 1%/100 mV for typical recordings. The root mean square noise of the apparatus (limited by shot noise) was typically 0.03%, equivalent to 3 mV of membrane potential. Illumination for at least 100 flashes of 100 ms each caused no noticeable photodynamic damage. Our results show that voltage-sensitive dyes can be used to record from microcultures of vertebrate neurons with high sensitivity. Dye signals were detected from both cell bodies and neurites. Signals from presumptive dendrites showed hyperpolarizations and action potentials simultaneous with those in the cell body, while those from presumptive axons showed delayed propagating action potentials. Subthreshold synaptic potentials in the cell body were occasionally detectable optically; however, they were usually masked by signals from axons passing through the same pixel. This is due to the complex anatomy of SCG microcultures, which have many crisscrossing neurites that often pass over cell bodies. Given a simpler microculture system with fewer neurites, it should be possible to use dye recording to routinely measure subthreshold synaptic strengths.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. H., Chapman P. F., Kairiss E. W., Keenan C. L. Long-term synaptic potentiation. Science. 1988 Nov 4;242(4879):724–728. doi: 10.1126/science.2903551. [DOI] [PubMed] [Google Scholar]
- Burton L. E., Wilson W. H., Shooter E. M. Nerve growth factor in mouse saliva. Rapid isolation procedures for and characterization of 7 S nerve growth factor. J Biol Chem. 1978 Nov 10;253(21):7807–7812. [PubMed] [Google Scholar]
- Chien C. B., Pine J. An apparatus for recording synaptic potentials from neuronal cultures using voltage-sensitive fluorescent dyes. J Neurosci Methods. 1991 Jul;38(2-3):93–105. doi: 10.1016/0165-0270(91)90159-w. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Lesher S. Optical monitoring of membrane potential: methods of multisite optical measurement. Soc Gen Physiol Ser. 1986;40:71–99. [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M., Davila H. V., Ross W. N., Landowne D., Waggoner A. S., Wang C. H. Changes in axon fluorescence during activity: molecular probes of membrane potential. J Membr Biol. 1974;19(1):1–36. doi: 10.1007/BF01869968. [DOI] [PubMed] [Google Scholar]
- Fluhler E., Burnham V. G., Loew L. M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry. 1985 Oct 8;24(21):5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- Freschi J. E. Membrane currents of cultured rat sympathetic neurons under voltage clamp. J Neurophysiol. 1983 Dec;50(6):1460–1478. doi: 10.1152/jn.1983.50.6.1460. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., Landis S. C., Matsumoto S. G., Potter D. D. Synaptic functions in rat sympathetic neurons in microcultures. I. Secretion of norepinephrine and acetylcholine. J Neurosci. 1986 Apr;6(4):1061–1079. doi: 10.1523/JNEUROSCI.06-04-01061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan E. J., MacLeish P. R., O'Lague P. H., Potter D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. B., Nyirjesy P., Pratap P. R., Freedman J. C., Waggoner A. S. Impermeant potential-sensitive oxonol dyes: III. The dependence of the absorption signal on membrane potential. J Membr Biol. 1988 Oct;105(1):55–64. doi: 10.1007/BF01871106. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Anglister L., Freeman J. A., Hildesheim R., Manker A. Real-time optical imaging of naturally evoked electrical activity in intact frog brain. 1984 Apr 26-May 2Nature. 308(5962):848–850. doi: 10.1038/308848a0. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Fine A., Farber I. C., Hildesheim R. Fluorescence monitoring of electrical responses from small neurons and their processes. Biophys J. 1983 May;42(2):195–198. doi: 10.1016/S0006-3495(83)84386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Frostig R. D., Lieke E., Hildesheim R. Optical imaging of neuronal activity. Physiol Rev. 1988 Oct;68(4):1285–1366. doi: 10.1152/physrev.1988.68.4.1285. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Hildesheim R., Farber I. C., Anglister L. Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J. 1982 Sep;39(3):301–308. doi: 10.1016/S0006-3495(82)84520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Salzberg B. M., Lev-Ram V., Hildesheim R. Optical recording of synaptic potentials from processes of single neurons using intracellular potentiometric dyes. Biophys J. 1987 Apr;51(4):643–651. doi: 10.1016/S0006-3495(87)83389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. D., Bodnar D. A., Kater S. B. Formation of electrical synapses between isolated, cultured Helisoma neurons requires mutual neurite elongation. J Neurosci. 1985 Dec;5(12):3145–3153. doi: 10.1523/JNEUROSCI.05-12-03145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D., Iacovitti L., Burton H. Fetal rat sympathetic neurons maintained in a serum-free medium retain induced cholinergic characteristics. Brain Res. 1984 May;316(1):71–82. doi: 10.1016/0165-3806(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D., Raccuia-Behling F., Chiel H. J. Circuits constructed from identified Aplysia neurons exhibit multiple patterns of persistent activity. Biophys J. 1990 Apr;57(4):697–715. doi: 10.1016/S0006-3495(90)82591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthamer V., Ross W. N. Regional variations in excitability of barnacle neurons. J Neurosci. 1984 Mar;4(3):673–682. doi: 10.1523/JNEUROSCI.04-03-00673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew L. M., Cohen L. B., Salzberg B. M., Obaid A. L., Bezanilla F. Charge-shift probes of membrane potential. Characterization of aminostyrylpyridinium dyes on the squid giant axon. Biophys J. 1985 Jan;47(1):71–77. doi: 10.1016/S0006-3495(85)83878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum D. K., Lillie J. H., Scaletta L. J., Occhino J. C., Frederick W. G., Ledbetter S. R. Bovine corneal endothelium in vitro. Elaboration and organization and of a basement membrane. Exp Cell Res. 1982 May;139(1):1–13. doi: 10.1016/0014-4827(82)90313-5. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Kleinfeld D., Raccuia-Behling F., Salzberg B. M. Optical recording of the electrical activity of synaptically interacting Aplysia neurons in culture using potentiometric probes. Biophys J. 1989 Jul;56(1):213–221. doi: 10.1016/S0006-3495(89)82666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D. D., Landis S. C., Matsumoto S. G., Furshpan E. J. Synaptic functions in rat sympathetic neurons in microcultures. II. Adrenergic/cholinergic dual status and plasticity. J Neurosci. 1986 Apr;6(4):1080–1098. doi: 10.1523/JNEUROSCI.06-04-01080.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Nicholls J. Identified neurones isolated from leech CNS make selective connections in culture. Nature. 1979 Sep 6;281(5726):67–69. doi: 10.1038/281067a0. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Pine J., Cohan C. S., Mischke M. D., Tank D. W. Sealing cultured invertebrate neurons to embedded dish electrodes facilitates long-term stimulation and recording. J Neurosci Methods. 1989 Nov;30(2):91–106. doi: 10.1016/0165-0270(89)90055-1. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Reichardt L. F. Species-specific effects on the optical signals of voltage-sensitive dyes. J Membr Biol. 1979 Aug;48(4):343–356. doi: 10.1007/BF01869445. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Grinvald A., Cohen L. B., Davila H. V., Ross W. N. Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. J Neurophysiol. 1977 Nov;40(6):1281–1291. doi: 10.1152/jn.1977.40.6.1281. [DOI] [PubMed] [Google Scholar]
- Syed N. I., Bulloch A. G., Lukowiak K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea. Science. 1990 Oct 12;250(4978):282–285. doi: 10.1126/science.2218532. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Wang C. H., Tolles R. L. Mechanism of potential-dependent light absorption changes of lipid bilayer membranes in the presence of cyanine and oxonol dyes. J Membr Biol. 1977 May 6;33(1-2):109–140. doi: 10.1007/BF01869513. [DOI] [PubMed] [Google Scholar]