Abstract
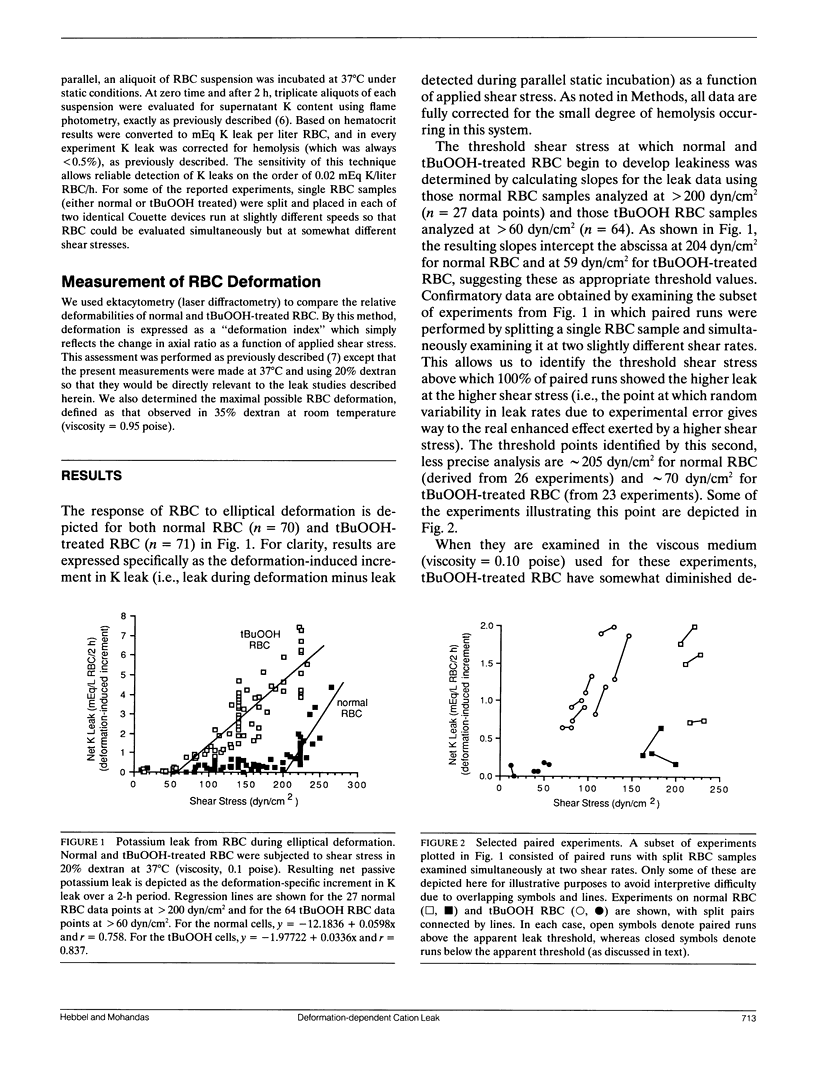
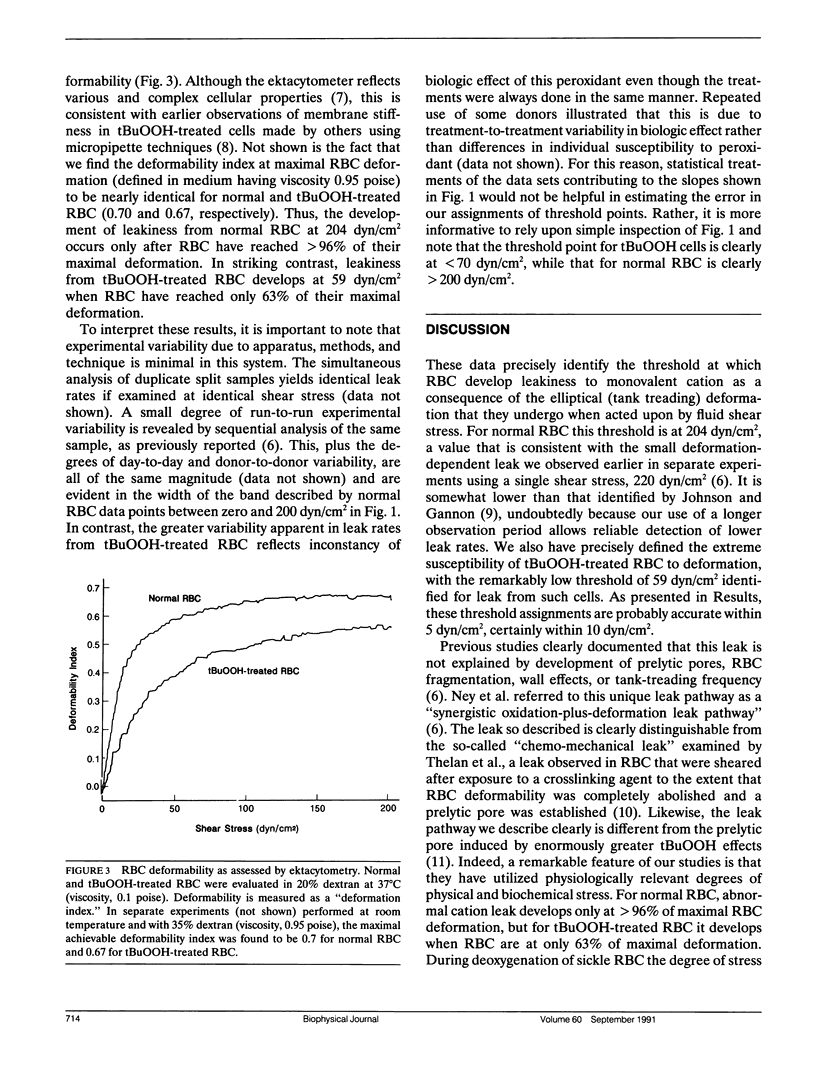
To determine the threshold at which red blood cells (RBC) begin to manifest deformation-dependent leakiness to monovalent cations, we examined net passive potassium leak during elliptical deformation. Normal RBC did not begin to leak appreciable amounts of potassium until shear stress reached 204 dyn/cm2, at which point they had attained greater than 96% of their maximal deformation. In striking contrast, RBC that had undergone minimal, physiologically relevant degrees of peroxidative damage induced by t-butylhydroperoxide began to leak potassium at only 59 dyn/cm2 when they had reached only 63% of their maximal deformation. The cation leak identified in this manner is not prelytic, and it is fully reversible. Therefore, these data may be relevant to abnormal cation leaks that develop in sickle red cells that have membranes damaged by auto-oxidative stress and that manifest an exuberant but reversible leakiness to monovalent cation during sickling-induced deformation of the cell membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corry W. D., Meiselman H. J., Hochstein P. t-Butyl hydroperoxide-induced changes in the physicochemical properties of human erythrocytes. Biochim Biophys Acta. 1980 Apr 10;597(2):224–234. doi: 10.1016/0005-2736(80)90101-7. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Heller K. B., Haest C. W. Leak formation in human erythrocytes by the radical-forming oxidant t-butylhydroperoxide. Biochim Biophys Acta. 1986 Jan 29;854(2):169–183. doi: 10.1016/0005-2736(86)90108-2. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991 Jan 15;77(2):214–237. [PubMed] [Google Scholar]
- Hebbel R. P. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin Hematol. 1990 Jan;27(1):51–69. [PubMed] [Google Scholar]
- Johnson R. M., Gannon S. A. Erythrocyte cation permeability induced by mechanical stress: a model for sickle cell cation loss. Am J Physiol. 1990 Nov;259(5 Pt 1):C746–C751. doi: 10.1152/ajpcell.1990.259.5.C746. [DOI] [PubMed] [Google Scholar]
- Larsen F. L., Katz S., Roufogalis B. D., Brooks D. E. Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature. 1981 Dec 17;294(5842):667–668. doi: 10.1038/294667a0. [DOI] [PubMed] [Google Scholar]
- Leverett L. B., Hellums J. D., Alfrey C. P., Lynch E. C. Red blood cell damage by shear stress. Biophys J. 1972 Mar;12(3):257–273. doi: 10.1016/S0006-3495(72)86085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubowitz H., Harris F., Mehrjardi M. H., Sutera S. P. Shear-induced changes in permeability of human RBC to sodium. Trans Am Soc Artif Intern Organs. 1974;20 B:470–473. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Christopher M. M., Hebbel R. P. Synergistic effects of oxidation and deformation on erythrocyte monovalent cation leak. Blood. 1990 Mar 1;75(5):1192–1198. [PubMed] [Google Scholar]
- Sugihara T., Rawicz W., Evans E. A., Hebbel R. P. Lipid hydroperoxides permit deformation-dependent leak of monovalent cation from erythrocytes. Blood. 1991 Jun 15;77(12):2757–2763. [PubMed] [Google Scholar]
- Sutera S. P. Flow-induced trauma to blood cells. Circ Res. 1977 Jul;41(1):2–8. doi: 10.1161/01.res.41.1.2. [DOI] [PubMed] [Google Scholar]
- Thelen P., Deuticke B. Chemo-mechanical leak formation in human erythrocytes upon exposure to a water-soluble carbodiimide followed by very mild shear stress. I. Basic characteristics of the process. Biochim Biophys Acta. 1988 Oct 6;944(2):285–296. doi: 10.1016/0005-2736(88)90443-9. [DOI] [PubMed] [Google Scholar]


