Abstract
Depletion of calcium ions (Ca2+) from the endoplasmic reticulum (ER) of yeast cells resulted in the activation of the unfolded protein response (UPR) signaling pathway involving Ire1p and Hac1p. The depleted ER also stimulated Ca2+ influx at the plasma membrane through the Cch1p–Mid1p Ca2+ channel and another system. Surprisingly, both Ca2+ influx systems were stimulated upon accumulation of misfolded proteins in the ER even in the presence of Ca2+. The ability of misfolded ER proteins to stimulate Ca2+ influx at the plasma membrane did not require Ire1p or Hac1p, and Ca2+ influx and signaling factors were not required for initial UPR signaling. However, activation of the Ca2+ channel, calmodulin, calcineurin and other factors was necessary for long-term survival of cells undergoing ER stress. A similar calcium cell survival (CCS) pathway operates in the pathogenic fungi and promotes resistance to azole antifungal drugs. These findings reveal an unanticipated new regulatory mechanism that couples ER stress to Ca2+ influx and signaling pathways, which help to prevent cell death and promote resistance to an important class of fungistatic drugs.
Keywords: Candida albicans/capacitative calcium entry/cell death/ER stress/Saccharomyces cerevisiae
Introduction
The endoplasmic reticulum (ER) of mammalian cells accumulates high levels of Ca2+ from the cytoplasm through the action of high-affinity Ca2+ pumps of the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) family (Moller et al., 1996; MacLennan et al., 1997). This pool of Ca2+ is important for the translocation, folding, glycosylation, disulfide bonding and sorting of secretory proteins in the ER through its direct interactions with a variety of ER-resident molecular chaperones and enzymes (Meldolesi and Pozzan, 1998). Agents that cause sustained depletion of Ca2+ from the ER, such as Ca2+ ionophores and chelators, inhibitors of SERCA pumps and activators of Ca2+ release channels, can all affect the efficiency of protein folding in the ER and thereby stimulate the unfolded protein response (UPR) signaling pathways (Lamarche et al., 1985; Drummond et al., 1987; Price et al., 1992; Gill et al., 1996). UPR signaling in mammalian cells involves the transmembrane protein kinases Ire1α, Ire1β and PERK, which dimerize and become activated in response to the accumulation of unfolded proteins in the ER (Shi et al., 1998; Tirasophon et al., 1998; Wang et al., 1998; Harding et al., 1999). The activated Ire1α and Ire1β kinases induce the transcription of the genes encoding ER-resident molecular chaperones and inhibit the translation of mRNAs encoding secretory proteins (Harding et al., 1999; Kaufman, 1999). Therefore, activation of UPR signaling pathways serves to enhance ER functioning on demand.
A second response to depletion of Ca2+ from the ER is the stimulation of Ca2+ influx through the plasma membrane, a process known as store-operated or capacitative Ca2+ entry (CCE) (Putney, 1986, 1990). This process serves to replenish the Ca2+-depleted organelles and to activate calcium signaling pathways. The molecular mechanisms by which plasma membrane Ca2+ channels become activated upon depletion of Ca2+ from the ER are not yet clear.
Recent evidence indicates that yeast may employ a CCE-like mechanism that helps to replenish secretory organelles with Ca2+ and to activate calcium signaling pathways (Locke et al., 2000). Yeast cells lack homologs of SERCA-type Ca2+ pumps, but express Pmr1p, a homolog of mammalian secretory pathway Ca2+ ATPases (SPCAs) (Rudolph et al., 1989; Antebi and Fink, 1992; Strayle et al., 1999). Pmr1p localizes to the Golgi complex and is important for supplying the Ca2+ and Mn2+ required for efficient glycosylation, processing and sorting reactions in this compartment (Dürr et al., 1998). Like mammalian cells treated with inhibitors of SERCA, pmr1 null mutants exhibit high rates of Ca2+ influx via a high-affinity Ca2+ channel composed of Cch1p and Mid1p (Locke et al., 2000). Cch1p is homologous to the catalytic subunit of voltage-gated Ca2+ channels in animals and, together with a putative regulatory subunit Mid1p, forms a Ca2+ influx pathway that can also be stimulated during the mating response (Iida et al., 1994; Fischer et al., 1997; Paidhungat and Garrett, 1997; Muller et al., 2001). The elevated Ca2+ influx in pmr1 mutants activates the calcineurin-dependent transcription factor Tcn1p (Matheos et al., 1997; Stathopoulos and Cyert, 1997; Mendizabal et al., 1998), which induces Pmc1p, a homolog of plasma membrane Ca2+ ATPases (PMCAs) localized to the vacuole (Cunningham and Fink, 1994, 1996; Halachmi and Eilam, 1996; Marchi et al., 1999). All these phenotypes of pmr1 mutants can be suppressed by expression of mammalian SERCA1a (Degand et al., 1999; Locke et al., 2000). Together, these findings indicate that Pmr1p is the functional equivalent of mammalian SERCA and that the Cch1p–Mid1p Ca2+ channel can become activated by a CCE-like mechanism.
Roles for Ca2+ in the yeast ER have been difficult to elucidate. The yeast calnexin homolog appears to function well without Ca2+ (Parlati et al., 1995). Additionally, free Ca2+ in the lumen of the yeast ER is 10–100 times lower than that of mammalian ER (Strayle et al., 1999). Mutants lacking Pmr1p accumulated half as much Ca2+ in the ER, but this decrease failed to stimulate UPR signaling (Dürr et al., 1998), indicating that high lumenal Ca2+ may not be required for protein folding in the ER of yeast. Because SERCA homologs have been identified in animals, plants, protozoa and even other fungi (Burk et al., 1989), the loss of SERCA-like enzymes in the evolution of budding yeast may reflect decreased necessity for Ca2+ in ER folding reactions. Alternatively, other Ca2+ ATPases such as Pmr1p, Pmc1p or possibly the newly identified P-type ATPase Cod1p/Spf1p (Cronin et al., 2000) may have compensated for the ancient loss of SERCA in yeast.
Here, we re-examined the roles of Ca2+ in the yeast ER using more stringent conditions and confirm that Ca2+ accumulation in the ER can enhance protein folding there much as in mammalian cells. Surprisingly, inhibitors of either protein folding or ergosterol biosynthesis in the ER were sufficient to stimulate Ca2+ influx and/or activate calcium signaling pathways that were essential for cell survival. This calcium cell survival (CCS) pathway was also active in the pathogenic yeasts Candida albicans and Candida glabrata, where it provides resistance to common antifungal drugs. Thus, existing antifungal therapies might be improved by blocking the CCS pathway with drugs that inhibit either the mechanism linking ER stress to Ca2+ influx or the essential targets of calcineurin.
Results
Activation of the UPR upon depletion of secretory Ca2+ stores
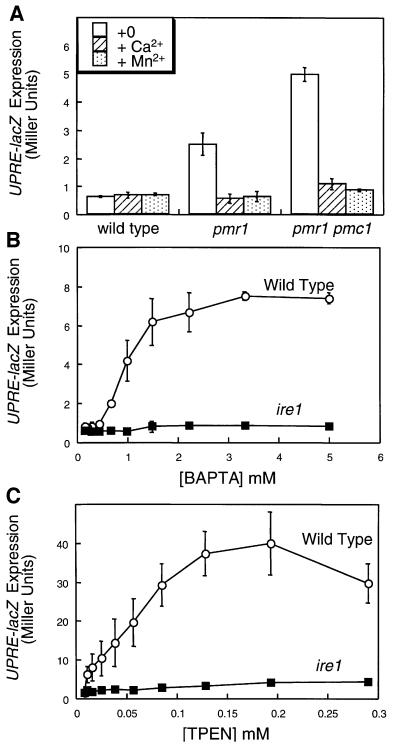
Mutants lacking the secretory pathway Ca2+ ATPase Pmr1p exhibit only mild defects in the sorting and processing of secretory proteins and no significant activation of UPR signaling during growth in standard media (Dürr et al., 1998). However, such conditions permit increased Ca2+ influx from the medium along with up-regulation and mislocalization of the vacuolar Ca2+ ATPase Pmc1p (Marchi et al., 1999; Locke et al., 2000), which might partially replenish secretory Ca2+ and thus compensate for the loss of Pmr1p. To address this possibility, we measured expression of UPRE-lacZ, a reporter of UPR signaling, in pmr1 pmc1 double mutants and in pmr1 mutants grown in medium deficient in Ca2+. Under these conditions, UPRE-lacZ expression was induced in pmr1 mutants and in pmr1 pmc1 double mutants but not in wild-type yeast cells (Figure 1A). Induction of UPRE-lacZ in the mutants was suppressed by supplementing the medium with high concentrations of CaCl2 or MnCl2 (Figure 1A), by expression of rabbit SERCA1a, or by disruption of the UPR signaling factor Ire1p (data not shown). Thus, genetic depletion of Ca2+ and Mn2+ from the secretory pathway led to activation of UPR signaling.
Fig. 1. Ca2+ starvation and chelation can stimulate the UPR signaling pathway. (A) Wild-type, pmr1 mutant and pmr1 pmc1 double mutant cells (strains K601, K609 and K613, respectively) bearing the UPRE-lacZ reporter gene (plasmid pCZY1) were grown to log phase in SC medium lacking uracil, Ca2+ and Mn2+ with or without a supplement of 10 mM CaCl2 or 100 µM MnCl2. After 4 h, the cells were harvested and assayed for β-galactosidase activity. Bars represent the mean of three independent transformants (± SD). The Pmr1p Ca2+ pump prevented activation of the UPR signaling pathway in medium lacking Ca2+. (B and C) Wild-type and ire1 mutant cells (strains K1257 and K1259, respectively) bearing the UPRE-lacZ reporter gene were grown to log phase in SC medium lacking uracil and containing 50 mM K-MES buffer pH 6.5, and then treated with the indicated concentrations of either the high-affinity membrane-impermeant Ca2+ chelator BAPTA or the low-affinity membrane-permeant Ca2+ chelator TPEN. Cells were incubated and processed for β-galactosidase activity as in (A).
Chelators of Ca2+, Mn2+ and other metal ions were also able to induce UPRE-lacZ expression in wild-type cells. Cells treated with either the membrane-impermeant chelator bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) (Figure 1B) or the membrane-permeant chelator N,N,N′,N′′-tetrakis-(2-pyridylmethyl) ethylenediamine (TPEN) (Figure 1C) exhibited high levels of expression of UPRE-lacZ (Figure 1B). In both cases, UPRE-lacZ induction was partially suppressed by overexpression of Pmr1p (data not shown) and abolished in ire1 mutants (Figure 1B and C). Taken together, these results provide evidence for a role of Ca2+ and the intracellular Ca2+ pump Pmr1p in preventing activation of the UPR, presumably by promoting the activities of Ca2+-dependent chaperones in the ER.
TPEN treatment stimulates Ca2+ influx and signaling
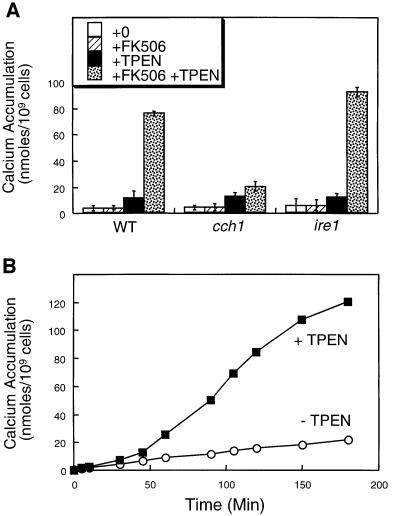
In cultured mammalian cells, TPEN treatment rapidly diminishes ER free Ca2+ concentration ([Ca2+]er) and activates the CCE pathway, which leads to elevation of cytosolic free Ca2+ concentration ([Ca2+]c) within 1 min of application (Hofer et al., 1998). We have previously reported evidence of a CCE-like pathway in yeast (Locke et al., 2000), but the use of genetic methods prevented a kinetic analysis of the CCE-like response. Therefore, we tested whether TPEN addition could stimulate Ca2+ influx and [Ca2+]c elevation in yeast on a time scale similar to that observed in mammalian cells. TPEN treatment stimulated 45Ca2+ accumulation in wild-type cells ∼10-fold in the presence of the calcineurin inhibitor FK506 and ∼2.5-fold in the absence of FK506 (Figure 2A). FK506 was used because it prevents calcineurin-dependent feedback inhibition of the Cch1p–Mid1p Ca2+ channel (Locke et al., 2000; Muller et al., 2001). Mutants lacking Cch1p exhibited a markedly diminished response to TPEN in the presence of FK506 (Figure 2A), indicating that TPEN may activate this Ca2+ channel in much the same way as a pmr1 mutation. Mutants lacking Ire1p or Hac1p exhibited a wild-type response to TPEN (Figure 2A and data not shown). Thus, TPEN appears to phenocopy pmr1 mutants and to trigger the CCE-like pathway in yeast, which is independent of UPR signaling.
Fig. 2. TPEN stimulates Ca2+ influx via the Cch1p–Mid1p Ca2+ channel independently of Ire1p. (A) 45Ca2+ accumulation in wild-type cells, cch1 mutants and ire1 mutants (strains K1257, RG05040 and K1259 respectively) was measured after incubation for 4 h in SC medium supplemented with trace amounts of 45CaCl2 plus 2.0 µg/ml FK506 and 129 µM TPEN as indicated. Bars represent the mean of three independent experiments (± SD). (B) 45Ca2+ accumulation in wild-type cells (strain K601) was measured at various time-points after treatment with either FK506 (open symbols) or TPEN plus FK506 (filled symbols) as in (A).
In time course experiments, the effect of TPEN treatment on 45Ca2+ accumulation became apparent after a lag of at least 30 min and reached a maximum after ∼150 min (Figure 2B). Similar kinetics were observed using cytoplasmic aequorin as a probe for [Ca2+]c (data not shown). Therefore, the Ca2+ influx observed in response to TPEN appears to be much slower in yeast relative to mammalian cells.
Misfolded secretory proteins stimulate Ca2+ influx and signaling
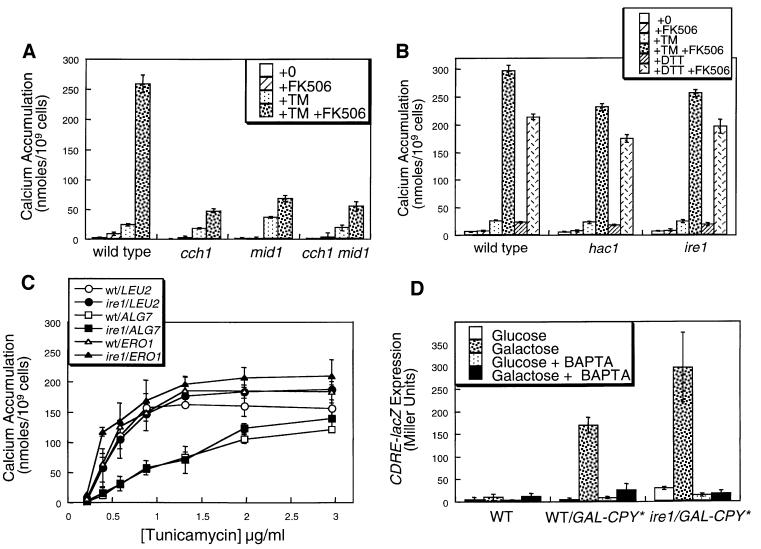
The relatively slow response of yeast cells to TPEN indicates that these cells may utilize a distinct mechanism for coupling Ca2+ influx to depletion of [Ca2+]er. To test whether this coupling involves the accumulation of misfolded proteins, we measured 45Ca2+ accumulation in cells treated with tunicamycin (TM), an inhibitor of N-glycosylation in the ER (Tkacz and Lampen, 1975; Lehle and Tanner, 1976) that causes accumulation of misfolded proteins and stimulation of UPR signaling. TM treatment significantly stimulated 45Ca2+ accumulation in wild-type cells and in cch1 mutants, mid1 mutants and cch1 mid1 double mutants (Figure 3A), suggesting the existence of a Ca2+ influx pathway that is independent of Cch1p–Mid1p. In the presence of FK506, TM stimulated even greater levels of 45Ca2+ accumulation, which was abolished in cch1 and mid1 mutants (Figure 3A). These effects of TM were completely independent of Ire1p and Hac1p (Figure 3B) but were clearly diminished in cells overexpressing Alg7p, the enzyme in the ER that is the direct target of TM (Figure 3C). The time course of Ca2+ accumulation in response to TM was similar to that of TPEN (data not shown). These findings indicate that specific inhibition of Alg7p and N-glycosylation in the ER can activate two Ca2+ influx systems through a mechanism that is independent of Ire1p and Hac1p. Identical results were obtained using dithiothreitol (DTT) as an inhibitor of disulfide bond formation in the ER and its target Ero1p (Figure 3B and data not shown). Thus, multiple inhibitors of protein folding or processing in the ER (e.g. TPEN, TM and DTT) all stimulated Ca2+ accumulation independently of a functional UPR signaling pathway.
Fig. 3. Accumulation of misfolded proteins in the ER stimulates Ca2+ influx and signaling independently of Ire1p. (A) 45Ca2+ accumulation in wild type, cch1 and mid1 mutants was measured as in Figure 2A except that TM (2.5 µg/ml) was used instead of TPEN. (B) 45Ca2+ accumulation in wild type, ire1 and hac1 mutants (strains K1257, K1259 and RG05650) was measured as in (A) except that DTT (2.5 mM) was included as indicated. (C) 45Ca2+ accumulation in wild-type (open symbols) and ire1 mutant cells (closed symbols) that were overexpressing Alg7p, Ero1p or no additional proteins in the ER (plasmids pMK13XRV, pKT001 or vector control) was measured after treatment with varying amounts of TM in the presence of 2 µg/ml FK506. Complementary results were obtained using DTT instead of TM (data not shown). (D) Expression of the CDRE-lacZ reporter gene (plasmid pAMS366) was measured in wild-type cells and ire1 mutants expressing a folding-incompetent mutant of carboxypeptidase Y (denoted CPY*) under control of the galactose promoter (strains DNY1047 and DNY1048) after growth for 4 h in SC medium lacking uracil but supplemented with either 2% glucose or 2% galactose as indicated. Induction of CPY* in galactose medium stimulated CDRE-lacZ expression through a process that was sensitive to both 4 mM BAPTA and FK506 (data not shown). Wild-type cells lacking CPY* (strain K601) were included as a control. Bars represent the mean of three independent transformants (± SD).
To test whether accumulation of a misfolded protein in the ER is sufficient to stimulate Ca2+ responses, the effects of overexpressing a folding-incompetent mutant of carboxypeptidase Y termed CPY* (Hiller et al., 1996) were measured. Overexpression of CPY* in wild-type or ire1 mutants stimulated 45Ca2+ accumulation (data not shown) and [Ca2+]c elevation as assessed by monitoring expression of a calcineurin-dependent reporter gene CDRE-lacZ (Figure 3C). Thus, accumulation of a single misfolded protein species in the ER appeared to be sufficient to stimulate Ca2+ influx and to activate calcineurin signaling. These findings indicate the existence of a novel UPR-independent regulatory pathway emanating from the ER that can sense the accumulation of misfolded proteins and stimulate Ca2+ influx at the plasma membrane as well as calcineurin signaling.
Roles for Ca2+ influx and signaling during the response to unfolded protein accumulation
What are the roles of Ca2+ during the response to ER stress? One possibility is that UPR signaling itself may depend on Ca2+ influx or signaling. Consistent with this possibility, UPRE-lacZ induction was 5-fold lower in response to BAPTA relative to TPEN, the former of which prevents [Ca2+]c elevation (Figures 1 and 2). To determine whether UPR signaling might involve calcium signaling factors, several assays of UPR signaling were performed on a panel of yeast strains lacking either Ca2+/calmodulin (cmd1-6 mutants), Ca2+/calmodulin-dependent protein kinases (cmk1 cmk2 double mutants), calcineurin (cnb1 mutants), calcineurin-dependent transcription factor (tcn1 mutants) or combinations thereof.
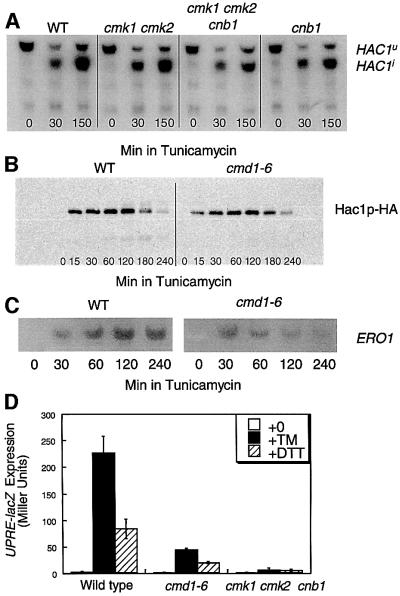
Early events in the UPR signaling pathway include the Ire1p-dependent splicing of the immature HAC1u pre-mRNA to the mature HAC1i mRNA, which is efficiently translated to promote accumulation of the Hac1p transcription factor (Sidrauski and Walter, 1997). Northern blot analysis of HAC1 transcripts in wild type, cnb1 mutants, cmk1 cmk2 and cmk1 cmk2 cnb1 triple mutants revealed no significant difference in either the kinetics or extent of TM-induced HAC1 mRNA splicing (Figure 4A and data not shown), although the basal levels of HAC1 transcription were slightly decreased. Western blot analysis of a functional epitope-tagged Hac1p–HA also revealed no significant differences between cmd1-6 mutants, cmk1 cmk2 cnb1 triple mutants and wild-type cells after treatment with TM (Figure 4B and data not shown). Finally, northern blot analysis of ERO1 transcripts, a target of Hac1p that is highly induced by UPR signaling (Frand and Kaiser, 1998), was performed in wild type, cmd1-6 mutants and cmk1 cmk2 cnb1 triple mutants at various times after treatment with TM. At early time-points, ERO1 expression was strongly induced in all these strains but ERO1 expression was not fully maintained in cmd1-6 mutants or cmk1 cmk2 cnb1 triple mutants at later time-points (Figure 4C and data not shown). The induction of UPRE-lacZ was markedly decreased in cmd1-6 mutants and cmk1 cmk2 cnb1 triple mutants as measured after 4 h of TM treatment (Figure 4D). These results indicate that calcium signaling factors are not required for the initial stages of UPR signaling. However, calcium signaling factors may be important to sustain UPR signaling during long periods of TM treatment.
Fig. 4. Calcium signaling factors are not required for efficient UPR signaling. (A) Alternative splicing of HAC1 transcripts was stimulated by tunicamycin independently of calcium signaling factors. HAC1 splicing was monitored by northern blot analysis of total RNA isolated from wild-type (K601), cmk1 cmk2 (K481), cmk1 cmk2 cnb1 (K488) and cnb1 (K603) strains after 0, 30 or 150 min in 2.5 µg/ml TM. (B) Accumulation of Hac1p was stimulated by tunicamycin treatment independently of calcium signaling factors. Hac1p accumulation was monitored by western blotting of total cell protein extracted from wild-type (K601) cells and cmd1-6 mutants (JGY148) carrying epitope-tagged Hac1p–HA (plasmid pMB004) that had been treated with 2.5 µg/ml TM for the indicated periods of time. (C) Northern blot analysis of ERO1 gene expression was performed as in (B) on wild-type and cmd1-6 mutant cells at various times after treatment with TM. Mutants defective in calmodulin signaling reproducibly induced ERO1 transcripts much more transiently than wild-type cells. (D) UPRE-lacZ expression was measured in wild type (strain K601), cmd1-6 mutants (strain JGY148) and cmk1 cmk2 cnb1 triple mutants (strain K488) after 4 h growth in SC medium supplemented with either 2.5 mg/ml TM or 2.5 mM DTT as indicated.
Calcineurin signaling prevents cell death in TM
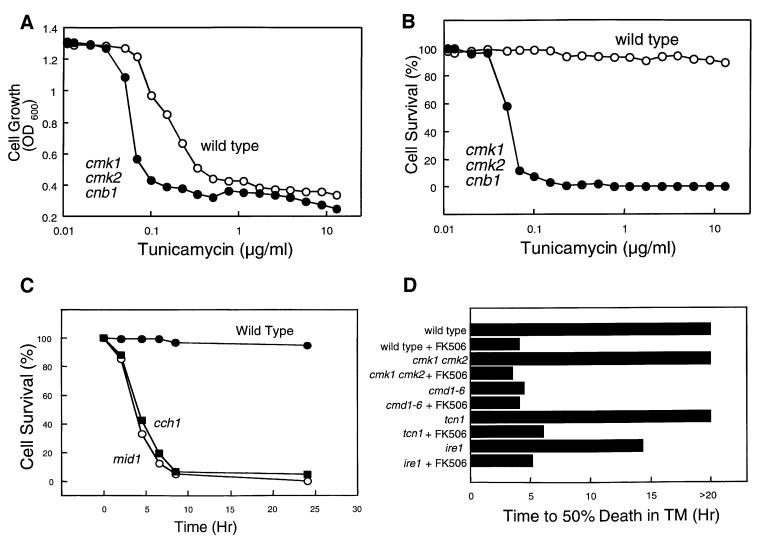
Wild-type yeast cells treated with TM remain viable for extended periods of time but fail to proliferate, apparently due to reversible arrest in either the G1 or G2 phase of the cell division cycle (Klebl et al., 1984). Mutants deficient in Alg7p behaved similarly (Lennon et al., 1997). We therefore explored the possible role of calcium signaling factors in cell growth and cell death after TM treatment. In growth assays (measurement of culture turbidity by optical density at 600 nm), cmk1 cmk2 cnb1 triple mutants were approximately four times more sensitive to TM than the wild-type strain (Figure 5A). However, in cell viability assays (exclusion of the vital dye methylene blue) wild-type cells remained almost completely viable after 6 h of treatment with TM whereas the cmk1 cmk2 cnb1 triple mutant cells exhibited striking levels of cell death (Figure 5B). In time course experiments, cch1 and mid1 mutants died rapidly in response to TM (Figure 5C). Therefore, Ca2+ influx and signaling factors were required for survival of arrested cells during prolonged treatment with TM.
Fig. 5. Tunicamycin treatment arrests growth of wild-type cells and causes death of cells deficient in Ca2+ influx and signaling factors. After treatment of log-phase yeast cultures with TM, dead cells were stained with methylene blue and counted. Cell survival reflects the percentage live cells of total cells in each culture. Wild type and cmk1 cmk2 cnb1 triple mutants (strains K601 and K488) were treated with varying amounts of TM for 6 h and then assayed for cell growth (A) or cell survival (B) as described in Materials and methods. (C) Survival of wild type, cch1 and mid1 mutants (strains K601, ELY117, ELY138) was measured at various times after addition of TM (2.5 µg/ml). (D) The time required for various yeast strains to undergo 50% cell death was determined in time course experiments similar to those in (C).
The relative contributions of individual calcium signaling factors were estimated by determining the time after TM treatment that resulted in death in 50% of various mutants. Wild-type cells and cmk1 cmk2 double mutants remained arrested but highly viable for at least 20 h of TM treatment but died rapidly if FK506 was also included (Figure 5D). In contrast, cmd1-6 mutants died rapidly with or without FK506. These findings indicate that cell survival requires activation of calcineurin by Ca2+/calmodulin. Mutants lacking the calcineurin-dependent transcription factor Tcn1p behaved like wild-type cells in survival assays (Figure 5D), indicating that calcineurin promotes cell survival independently of Tcn1p. Interestingly, Ire1p was not required for this role of calcineurin because ire1 mutants survived much longer without FK506 than with FK506 (Figure 5D). These findings demonstrate that calcium signaling factors such as Cch1p, Mid1p, calmodulin and calcineurin promote long-term survival of cells treated with TM and that this role is not shared with UPR signaling factors. This CCS pathway has not been described previously.
The CCS pathway is required for resistance to azole antifungal drugs and operates in pathogenic fungi
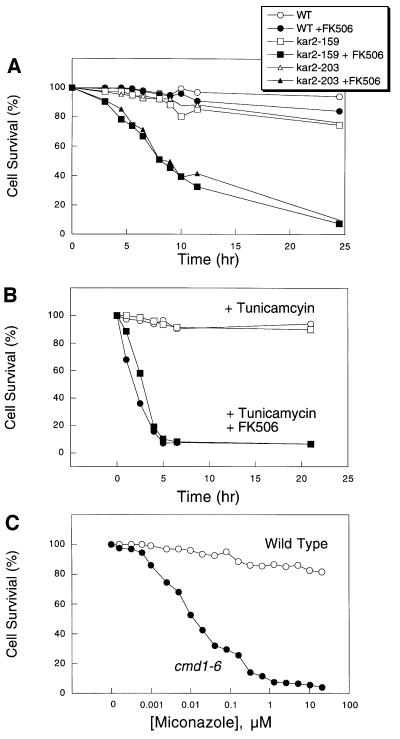
To appreciate the range of conditions that stimulate the CCS pathway, we tested several additional inhibitors and mutations that affect ER function. Like TM, addition of DTT caused much more rapid cell death in mutants lacking the calcium signaling factors in comparison with wild type (data not shown). To inhibit protein folding without the use of inhibitors such as TM or DTT, we examined the survival of temperature-sensitive kar2 mutants in which the ER-localized Hsp70-family chaperone homologous to mammalian BiP could be conditionally inactivated. After a shift from 23°C to the semi-permissive temperature of 30°C, two different kar2-ts mutants rapidly arrested growth relative to wild type, but remained viable for at least 24 h (Figure 6A). Viability of the mutants was rapidly lost at the semi-permissive temperature if FK506 was included (Figure 6A). Thus, genetic deficiencies in the ER protein folding machinery appear to require calcium signaling pathways for cell survival.
Fig. 6. Calcineurin signaling is required for survival of kar2-ts mutants and for survival of Saccharomyces and Candida species in response to azoles or other ER stress agents. (A) FK506 prevents survival of kar2-159 (strain MS137) and kar2-203 (strain MS1028) at a semi-permissive temperature (30°C). Cell survival assays were performed as described in Figure 5. (B) FK506 (filled symbols) prevents survival of C.albicans (circles) and C.glabrata (squares) in medium containing TM. (C) Calmodulin signaling was required for survival in medium containing miconazole. Survival of wild type and cmd1-6 mutants was measured after treatment with varying amounts of miconazole for 6 h in SC medium.
To test whether the CCS pathway might operate in other fungi, cell survival assays were performed on pathogenic strains of C.albicans and C.glabrata treated with TM and FK506. In both of these species, treatment with TM plus FK506 resulted in rapid cell death whereas treatment with TM alone arrested growth without causing cell death (Figure 6B). Treatment with FK506 alone had no effect on growth or death of these cells. Thus, the CCS pathway appears to operate in diverse fungi.
The cytotoxic effects of FK506 during TM treatment resemble the cytotoxic effects of FK506 observed in pathogenic strains of C.albicans that had been treated with azole-class antifungal drugs that inhibit ergosterol biosynthesis in the fungal ER (Marchetti et al., 2000). To determine whether the CCS pathway can provide tolerance to azole-class antifungal drugs in Saccharomyces, we measured the survival of wild-type and cmd1-6 mutant cells after a 6 h treatment with various concentrations of miconazole (Figure 6C). Wild-type yeast cells arrested but remained highly viable at concentrations of miconazole up to 20 µM whereas cmd1-6 mutant cells arrested and died at concentrations >0.2 µM. Miconazole treatment stimulated 45Ca2+ accumulation in wild-type cells, but did not stimulate expression of UPRE-lacZ (data not shown). These results indicate that the CCS pathway becomes activated in response to miconazole and promotes cell survival. The CCS pathway may therefore be an important determinant of cellular resistance to azole-class antifungal drugs.
Discussion
This report provides evidence that Ca2+ accumulation in the ER is important for folding or processing of secretory proteins in yeast, much as it is in other eukaryotes. Accumulation of unfolded proteins or inhibition of ergosterol synthesis in the ER of yeast was sufficient to stimulate Ca2+ influx via the Cch1p–Mid1p channel and other pathways, which promoted cell survival through the activation of calcineurin. The existence of this CCS pathway in fungi may provide new opportunities for treatment of fungal infections as well as a model system for studies of similar pathways in human cells.
Roles of Ca2+ in the ER
Until now, little evidence was available to support the notion that the ER of yeast employs Ca2+ for the folding or processing of secretory proteins. Previously it was shown that mutants lacking Pmr1p failed to retain a heterologous secretory protein in the ER (Rudolph et al., 1989) and failed to degrade misfolded proteins in the ER, although UPR signaling was not elevated (Dürr et al., 1998). Our findings indicate that high extracellular Ca2+ and consequent up-regulation of Pmc1p suppresses the loss of Pmr1p and thus obscures a role for Ca2+ in the modification or folding of secretory proteins. Inactivation of Pmc1p and/or removal of excess Ca2+ from the medium led to high UPR signaling in pmr1 mutants (Figure 1). Treatment with the extracellular chelator BAPTA or intracellular chelator TPEN caused similar effects in wild-type cells. Though these chelators are not specific for Ca2+, their effects were at least partially reversed by overexpression of Pmr1p or rabbit SERCA1a (not shown), indicating they deplete cells of a Pmr1p substrate. Mn2+ was effective at suppressing secretory defects of pmr1 mutants (Figure 1A), confirming an earlier observation that Mn2+ often substitutes for Ca2+ in the secretory pathway of yeast (Loukin and Kung, 1995).
Communication between the ER and plasma membrane
Most mammalian cell types monitor Ca2+ levels in the ER and rapidly activate the CCE mechanism in response to even transient Ca2+ depletion (Putney, 1986). For example, TPEN treatment depletes [Ca2+]er very rapidly, and within seconds the depleted organelle triggers CCE at the plasma membrane and elevation of [Ca2+]c (Hofer et al., 1998). In yeast, TPEN stimulated Ca2+ entry and elevated [Ca2+]c (Figure 2A), but these effects required at least 1 h of treatment (Figure 2B). The different kinetics might reflect a lower permeability of yeast cells to TPEN, or possibly a distinct mechanism in yeast cells for regulating Ca2+ entry such as ER stress. Several other agents and conditions that stimulate UPR signaling in yeast, including TM, DTT, CPY*, kar2-ts mutation and pmr1 mutation, also stimulated Ca2+ accumulation. Their effects on Ca2+ influx and signaling were completely independent of Ire1p and Hac1p, two crucial components of the UPR signaling pathway. Furthermore, an inhibitor of ergosterol biosynthesis in the ER also stimulated calcium signaling but did not activate UPR signaling (data not shown). These findings suggest that more general forms of ER stress can regulate Ca2+ entry in yeast. Though the signaling pathways employed to couple ER stress to Ca2+ influx remain to be identified, our results exclude the UPR signaling pathway.
All of the ER stress agents and conditions reported here appeared to stimulate two separate Ca2+ influx systems, one of which required Mid1p and Cch1p and the other remaining unidentified. Mid1p homologs are conserved in other fungi and are required for resistance to echinocandin-class drugs in Schizosaccharomyces pombe (Carnero et al., 2000; Tasaka et al., 2000). Cch1p is homologous to the catalytic subunit of voltage-gated Ca2+ channels (VGCCs) conserved in fungi and animals, but is most closely related to an uncharacterized family of four-repeat channels that is conserved in the genomes of nematodes, fruit flies and mammals (Lee et al., 1999). The possibility that ER stress activates these channels in mammalian cells has not been ruled out. In fact, ER stresses such as TM treatment or even Alzheimer’s disease mutations in presenilin-1 can affect CCE (Pahl and Baeuerle, 1996; Yoo et al., 2000) and can also affect the activity of Cch1p homologs that contribute to neuronal cell death and neurodegenerative disease (Paschen and Frandsen, 2001). Defining the signal transduction pathway that couples ER stress to Cch1p–Mid1p activation in yeast may therefore help to elucidate similar mechanisms in human cells.
Roles for Ca2+ influx in response to ER stress
Yeast mutants lacking Ca2+/calmodulin (cmd1-6) or its effectors (cmk1 cmk2 cnb1 triple mutants) appeared indistinguishable from wild-type cells in their ability to execute the early stages of UPR signaling (Figure 4). However, these mutants did not maintain long-term expression of target genes and largely failed to accumulate β-galactosidase activity expressed from the UPRE-lacZ reporter gene. These defects might reflect a role for Ca2+ and calcium signaling factors at a late step in the UPR signaling pathway. Alternatively, the inability to maintain UPR signaling may be a consequence of the dramatically accelerated cell death observed in the mutants during ER stress.
Yeast strains lacking Cch1p, Mid1p, calmodulin or calcineurin functions died rapidly in repsonse to ER stress agents whereas mutants lacking Tcn1p, the only known calcineurin-dependent transcription factor, remained fully viable for long periods of time, like wild-type cells (Figure 6). This Tcn1p-independent cell survival pathway appears to be distinct from the calcineurin-dependent growth of cells with cell wall defects (Mazur et al., 1995; Carnero et al., 2000), which requires Tcn1p (Matheos et al., 1997). Survival of yeast undergoing ER stress was also independent of Vcx1p and Hsl1p (M.Bonilla and K.W.Cunningham, unpublished results), two other suspected targets of calcineurin (Cunningham and Fink, 1996; Mizunuma et al., 2001). Because a similar pathway appears to operate in pathogenic fungi and promote resistance to the azole class of antifungal drugs (Figure 6; Del Poeta et al., 2000; Marchetti et al., 2000; Cruz et al., 2002), identification of the targets of calcineurin responsible for cell survival under these conditions may be useful in the development of new antifungal therapies.
Materials and methods
Yeast strains, plasmids and growth conditions
All yeast strains used in this study (Table I) were obtained from original sources or derived from the parental strain W303-1A (Wallis et al., 1989) or BY4741 (Brachmann et al., 1998) using standard molecular and genetic methods (Sambrook et al., 1989). The CMK1 and CMK2 genes were disrupted in wild-type strain K601 by transformation with fragments of plasmids pBKScmk1Δ1 and pBKScmk2Δ2 (Pausch et al., 1991) to yield strain K481 and a MATα derivative K523. The CNB1 gene was disrupted in strain K523 as described (Cyert and Thorner, 1992) to yield strain K488. Strains K1257 and K1259 (ire1::G418) were recovered after sporulation of strain RG21907 (Research Genetics, Inc.).
Table I. Fungal strains used in this study.
| Strain | Genotype | Source or reference |
|---|---|---|
| W303-1A | MATa ade2-1 can1-100 his3-11,14 leu2-3 112 trp1-1 ura3-1 | Wallis et al. (1989) |
| K609 | pmr1::HIS3 | Cunningham and Fink (1994) |
| K613 | pmr1::HIS3 pmc1::TRP1 | Cunningham and Fink (1994) |
| JGY148 | cmd1-6 | Geiser et al. (1991) |
| K481 | cmk1::HIS3 cmk2::TRP1 | This study |
| K488 | cmk1::HIS3 cmk2::TRP1 cnb1::LEU2 | This study |
| K603 | cnb1::LEU2 | Cunningham and Fink (1994) |
| DMY14 | tcn1::G418 | Matheos et al. (1997) |
| ELY117 | cch1::TRP1 | Locke et al. (2000) |
| ELY138 | mid1::LEU2 | Locke et al. (2000) |
| ELY151 | cch1::TRP1 mid1::LEU2 | Locke et al. (2000) |
| DNY1047 | LEU2::GAL-CPY* | Gift of Davis Ng |
| DNY1048 | LEU2::GAL-CPY* ire1::TRP1 | Gift of Davis Ng |
| BY4741 | MATα his3-1 leu2-0 ura3-0 | Brachmann et al. (1998) |
| RG05650 | BY4741 hac1::G418 | Research Genetics |
| RG05040 | BY4741 cnb1::G418 | Research Genetics |
| K1257 | BY4741 | This study |
| K1259 | BY4741 ire1::G418 | This study |
| MS10 | MATa ura3-52 leu2-3 leu2-112 ade2-101 | Vogel et al. (1990) |
| MS137 | MS10 kar2-159 | Sanders et al. (1992) |
| MS1028 | MS10 kar2-203 | Scidmore et al. (1993) |
| BG2 | C.glabrata wild type | Cormack and Falkow (1999) |
| Ca5314 | C.albicans wild type | Fonzi and Irwin (1993) |
Overexpression of Ero1p and Alg7p in yeast was achieved by transformation with high-dosage plasmids pKT001 (2µ URA3 ERO1) (Pollard et al., 1998) and pMK13×RV (2µ LEU2 ALG7) (gift of Maria Kukuruzinska), respectively. Activation of the UPR signaling pathway was monitored in yeast transformed with plasmid pCZY1 (2µ URA3 UPRE-lacZ) (Mori et al., 1996). Activation of the calcium signaling pathway was monitored in yeast transformed with pAMS366 (2µ URA3 4×CDRE-lacZ) (Stathopoulos and Cyert, 1997). Plasmid pMB004, which expresses epitope-tagged Hac1p–HA, was constructed by inserting two repeats of the 96 bp triple-tandem HA1 sequence (Field et al., 1988) into the SpeI site (at position +25 in the HAC1 open reading frame) of plasmid YCp-HAC1WT (Mori et al., 1996).
Yeast strains were grown in standard yeast peptone dextrose (YPD) medium, synthetic complete (SC) medium containing 2% glucose, galactose or raffinose, as described previously (Sherman et al., 1986), using reagents from Difco (Detroit, MI, USA) and Sigma Chemical Co. (St Louis, MO, USA). Calcium-free SD medium was made by replacing YNB with divalent cation-free yeast nitrogen base (Q-Biogene Inc.) and supplementing with the chloride salts of Cu2+, Fe2+, Mg2+ and Zn2+ (but not Mn2+ or Ca2+) to their standard concentrations. The Ca2+ chelators BAPTA and TPEN were obtained from Sigma, dissolved in water and dimethylsulfoxide (DMSO), respectively, and diluted into SC culture medium pH 6.5, supplemented with 50 mM MES–KOH, to final concentrations of 3 mM and 130 µM, respectively. TM (Sigma) was dissolved in methanol and diluted into medium to a final concentration of 2.5 µg/ml. DTT (Gibco/Life Technologies, Inc.) was dissolved in water and diluted into YPD medium to a final concentration of 2.5 mM. These concentrations were chosen based on their maximal stimulation of UPRE-lacZ expression in strain W303-1A.
β-galactosidase assays
Yeast strains transformed with plasmids carrying either the UPRE-lacZ or CDRE-lacZ reporter gene were grown to log phase in SC media (shaking overnight at 30°C), harvested by centrifugation, then cultured in SC medium containing the indicated concentrations of chelators or inhibitors. After incubation at 30°C for 4 h, cells were harvested by centrifugation, permeabilized with chloroform and assayed for β-galactosidase activity using the colorimetric substrate ONPG as described previously (Guarente, 1983). The effects of CPY* overexpression in wild-type (DNY1047) or ire1 mutant (DNY1048) cells were monitored after cells were grown to log phase in SC medium supplemented with 2% raffinose, washed twice, then shifted to SC medium supplemented with 2% galactose or 2% glucose for 4 h.
45Ca2+ accumulation measurements
Total cellular accumulation of Ca2+ was measured as described previously (Cunningham and Fink, 1996). Briefly, yeast cells were grown to log phase in YPD medium, harvested and resuspended in fresh YPD medium supplemented with tracer quantities (∼20 µCi/ml) of 45CaCl2 (Amersham). After 4 h incubation at 30°C, cells were harvested rapidly by filtration onto GF/F filters (Whatman), washed with ice-cold buffer W (10 mM CaCl2, 5 mM HEPES–NaOH pH 6.5), dried at 90°C for 1 h, placed in scintillation vials and processed for liquid scintillation counting on a Packard 1600 TR liquid scintillation counter using Opti-Fluor (Packard) scintillation cocktail.
Northern blot analysis
Yeast cells growing in YPD medium were treated with chelators or inhibitors for 20 min at 30°C, chilled on ice and harvested by centrifugation at 700 g at 4°C, for 5 min. Total RNA was extracted and electrophoresed through agarose gels essentially as described (Zhao et al., 1998). For northern blot analysis, 20 µg of total RNA was loaded and separated on a 1% agarose–formaldehyde gel [1% agarose, 20 mM morpholinepropanesulfonic acid (MOPS), 1 mM EDTA, 5 mM sodium acetate, 2.2 M formaldehyde]. RNA was blotted onto Hybond-N+ membrane by capillary transfer and cross-linked by UV radiation. Membranes were blocked, hybridized overnight at 42°C with ∼0.5 × 106 c.p.m./ml of [α-32P]dCTP-labelled DNA probe, washed and subsequently imaged by autoradiography using X-Omat film. Probes for detecting HAC1 and ERO1 mRNAs were synthesized by PCR amplification of wild-type yeast genomic DNA using the primer pairs AATTCGCAATCGAACTTGGC plus TATATCGTCGCAGAGTGGGT and AGATTAAGAACCGCCATTGC plus ATAGGCTCTCGTGTCTCCTCA, respectively, purified and labelled using a random primer kit (Gibco/Life).
Western blot analysis
Whole-cell extracts were prepared by glass bead lysis from ∼2 OD600 units of log-phase yeast cells carrying plasmid pMB004 (CEN LEU2 HAC1–HA). Cells were collected by centrifugation and resuspended in 40 µl of sorbitol breaking buffer (0.3 M sorbitol, 0.1 M NaCl, 5 mM MgCl2, 10 mM Tris pH 7.5, protease inhibitors) (Katzmann et al., 1999). Glass beads were added to the meniscus, and samples were vortexed at 4°C for 5 min in the presence of protease inhibitors [Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK), Nα-p-tosyl-l-arginine methyl ester (TAME), Nα-benzoyl-l-arginine methyl ester (BAME), N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK), 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF), leupeptin and pepstatin all from Sigma]. Sample buffer was added to whole-cell lysates and the lysates were loaded onto SDS–PAGE gels. After running gels, proteins were trans ferred to PVDF and membranes were probed with 12CA5 anti-myc monoclonal antibody (Santa Cruz). A secondary horseradish peroxidase-conjugated goat anti-mouse antibody (Jackson Immunoresearch) was used in conjunction with an ECL kit to visualize Hac1p–HA bands on the membranes.
Methylene blue viability assays
Log-phase cultures of yeast growing in SC medium were treated with tunicamcyin and/or FK506 and incubated at 30°C. At intervals, aliquots were removed and stained with 100 µg/ml methylene blue (Sigma). Live cells (unstained) and dead cells (blue stained) were immediately counted under bright-field microscopy. At least 200 live and dead cells were counted at each time-point and the data were plotted as the percentage of live cells.
Acknowledgments
Acknowledgements
We wish to thank Davis Ng, Kazutoshi Mori, Jonathan Weissman, Brendan Cormack, Mark Rose and Maria Kukuruzinska for providing plasmids and yeast strains used in this study, as well as Fujisawa USA for the generous gift of FK506. In addition, we thank all the members of our department and laboratory for discussion and advice on this project, as well as Elizabeth O’Sullivan and Christian Martin for expert technical assistance and Andrew Timmes for initial experimental results. This study was supported by grants from the DuPont Young Professors Award and the National Institutes of Health (GM53082).
References
- Antebi A. and Fink,G.R. (1992) The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell, 3, 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C.B., Davies,A., Cost,G.J., Caputo,E., Li,J., Hieter,P. and Boeke,J.D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast, 14, 115–132. [DOI] [PubMed] [Google Scholar]
- Burk S.E., Lytton,J., MacLennan,D.H. and Shull,G.E. (1989) cDNA cloning, functional expression and mRNA tissue distribution of a third organellar Ca2+ pump. J. Biol. Chem., 264, 18561–18568. [PubMed] [Google Scholar]
- Carnero E., Ribas,J.C., Garcia,B., Duran,A. and Sanchez,Y. (2000) Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet., 264, 173–183. [DOI] [PubMed] [Google Scholar]
- Cormack B.P. and Falkow,S. (1999) Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics, 151, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin S.R., Khoury,A., Ferry,D.K. and Hampton,R.Y. (2000) Regulation of HMG-CoA reductase degradation requires the P-type ATPase Cod1p/Spf1p. J. Cell Biol., 148, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M.C., Goldstein,A.L., Blankenship,J.R., Del Poeta,M., Davis,D., Cardenas,M.E., Perfect,J.R., McCusker,J.H. and Heitman,J. (2002) Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J., 21, 546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K.W. and Fink,G.R. (1994) Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol., 124, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K.W. and Fink,G.R. (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in yeast. Mol. Cell. Biol., 16, 2226–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M.S. and Thorner,J. (1992) Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol., 12, 3460–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degand I., Catty,P., Talla,E., Thinès-Sempoux,D., De Kerchove D’Exaerde,A., Goffeau,A. and Ghislain,M. (1999) Rabbit sarcoplasmic reticulum Ca2+-ATPase replaces yeast PMC1 and PMR1 Ca2+-ATPases for cell viability and calcineurin-dependent regulation of calcium tolerance. Mol. Microbiol., 31, 545–556. [DOI] [PubMed] [Google Scholar]
- Del Poeta M., Cruz,M.C., Cardenas,M.E., Perfect,J.R. and Heitman,J. (2000) Synergistic antifungal activities of bafilomycin A1, fluconazole and the pneumocandin MK-0991/caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob. Agents Chemother., 44, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I.A., Lee,A.S., Resendez,E.,Jr and Steinhardt,R.A. (1987) Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem., 262, 12801–12805. [PubMed] [Google Scholar]
- Dürr G., Strayle,J., Plemper,R., Elbs,S., Klee,S.K., Catty,P., Wolf,D.H. and Rudolph,H.K. (1998) The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell, 9, 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa,J., Broek,D., MacDonald,B., Rodgers,L., Wilson,I.A., Lerner,R.A. and Wigler,M. (1988) Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol. Cell. Biol., 8, 2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M., Schnell,N., Chattaway,J., Davies,P., Dixon,G. and Sanders,D. (1997) The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. FEBS Lett., 419, 259–262. [DOI] [PubMed] [Google Scholar]
- Fonzi W.A. and Irwin,M.Y. (1993) Isogenic strain construction and gene mapping in Candida albicans. Genetics, 134, 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand A.R. and Kaiser,C.A. (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell, 1, 161–170. [DOI] [PubMed] [Google Scholar]
- Geiser J.R., van Tuinen,D., Brockerhoff,S.E., Neff,M.M. and Davis,T.N. (1991) Can calmodulin function without binding calcium? Cell, 65, 949–959. [DOI] [PubMed] [Google Scholar]
- Gill D.L., Waldron,R.T., Rys-Sikora,K.E., Ufret-Vincenty,C.A., Graber,M.N., Favre,C.J. and Alfonso,A. (1996) Calcium pools, calcium entry and cell growth. Biosci. Rep., 16, 139–157. [DOI] [PubMed] [Google Scholar]
- Guarente L. (1983) Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol., 101, 181–191. [DOI] [PubMed] [Google Scholar]
- Halachmi D. and Eilam,Y. (1996) Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett., 392, 194–200. [DOI] [PubMed] [Google Scholar]
- Harding H.P., Zhang,Y. and Ron,D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 397, 271–274. [DOI] [PubMed] [Google Scholar]
- Hiller M.M., Finger,A., Schweiger,M. and Wolf,D.H. (1996) ER degradation of a misfolded luminal protein by the cytosolic ubiquitin–proteasome pathway. Science, 273, 1725–1728. [DOI] [PubMed] [Google Scholar]
- Hofer A.M., Fasolato,C. and Pozzan,T. (1998) Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+]. J. Cell Biol., 140, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Nakamura,H., Ono,T., Okumura,M.S. and Anraku,Y. (1994) MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol., 14, 8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann D.J., Epping,E.A. and Moye-Rowley,W.S. (1999) Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol. Cell. Biol., 19, 2998–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R.J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev., 13, 1211–1233. [DOI] [PubMed] [Google Scholar]
- Klebl F., Huffaker,T. and Tanner,W. (1984) A temperature-sensitive N-glycosylation mutant of S. cerevisiae that behaves like a cell-cycle mutant. Exp. Cell Res., 150, 309–313. [DOI] [PubMed] [Google Scholar]
- Lamarche S., Chretien,P. and Landry,J. (1985) Inhibition of the heat shock response and synthesis of glucose-regulated proteins in Ca2+-deprived rat hepatoma cells. Biochem. Biophys. Res. Commun., 131, 868–876. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Cribbs,L.L. and Perez-Reyes,E. (1999) Cloning of a novel four repeat protein related to voltage-gated sodium and calcium channels. FEBS Lett., 445, 231–236. [DOI] [PubMed] [Google Scholar]
- Lehle L. and Tanner,W. (1976) The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett., 72, 167–170. [DOI] [PubMed] [Google Scholar]
- Lennon K., Bird,A. and Kukuruzinska,M.A. (1997) Deregulation of the first N-glycosylation gene, ALG7, perturbs the expression of G1 cyclins and cell cycle arrest in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun., 237, 562–565. [DOI] [PubMed] [Google Scholar]
- Locke E.G., Bonilla,M., Liang,L., Takita,Y. and Cunningham,K.W. (2000) A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol., 20, 6686–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S. and Kung,C. (1995) Manganese effectively supports yeast cell-cycle progression in place of calcium. J. Cell Biol., 131, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D.H., Rice,W.J. and Green,N.M. (1997) The mechanism of Ca2+ transport by sarco(endo)plasmic reticulum Ca2+-ATPases. J. Biol. Chem., 272, 28815–28818. [DOI] [PubMed] [Google Scholar]
- Marchetti O., Moreillon,P., Glauser,M.P., Bille,J. and Sanglard,D. (2000) Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob. Agents Chemother., 44, 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi V., Sorin,A., Wei,Y. and Rao,R. (1999) Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett., 454, 181–186. [DOI] [PubMed] [Google Scholar]
- Matheos D.P., Kingsbury,T.J., Ahsan,U.S. and Cunningham,K.W. (1997) Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev., 11, 3445–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Morin,N., Baginsky,W., El-Sherbeini,M., Clemas,J.A., Nielsen,J.B. and Foor,F. (1995) Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol., 15, 5671–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J. and Pozzan,T. (1998) The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci., 23, 10–14. [DOI] [PubMed] [Google Scholar]
- Mendizabal I., Rios,G., Mulet,J.M., Serrano,R. and de Larrinoa,I.F. (1998) Yeast putative transcription factors involved in salt tolerance. FEBS Lett., 425, 323–328. [DOI] [PubMed] [Google Scholar]
- Mizunuma M., Hirata,D., Miyaoka,R. and Miyakawa,T. (2001) GSK-3 kinase Mck1 and calcineurin coordinately mediate Hsl1 down-regulation by Ca2+ in budding yeast. EMBO J., 20, 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller J.V., Juul,B. and le Maire,M. (1996) Structural organization, ion transport and energy transduction of P-type ATPases. Biochim. Biophys. Acta, 1286, 1–51. [DOI] [PubMed] [Google Scholar]
- Mori K., Kawahara,T., Yoshida,H., Yanagi,H. and Yura,T. (1996) Signalling from endoplasmic reticulum to nucleus: transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells, 1, 803–817. [DOI] [PubMed] [Google Scholar]
- Muller E.M., Locke,E.G. and Cunningham,K.W. (2001) Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics, 159, 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H.L. and Baeuerle,P.A. (1996) Activation of NF-κB by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett., 392, 129–136. [DOI] [PubMed] [Google Scholar]
- Paidhungat M. and Garrett,S. (1997) A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol., 17, 6339–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Dominguez,M., Bergeron,J.J. and Thomas,D.Y. (1995) Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus. J. Biol. Chem., 270, 244–253. [DOI] [PubMed] [Google Scholar]
- Paschen W. and Frandsen,A. (2001) Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? J. Neurochem., 79, 719–725. [DOI] [PubMed] [Google Scholar]
- Pausch M.H., Kaim,D., Kunisawa,R., Admon,A. and Thorner,J. (1991) Multiple Ca2+/calmodulin-dependent protein kinase genes in a unicellular eukaryote. EMBO J., 10, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard M.G., Travers,K.J. and Weissman,J.S. (1998) Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell, 1, 171–182. [DOI] [PubMed] [Google Scholar]
- Price B.D., Mannheim-Rodman,L.A. and Calderwood,S.K. (1992) Brefeldin A, thapsigargin and AIF4 stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J. Cell. Physiol., 152, 545–552. [DOI] [PubMed] [Google Scholar]
- Putney J.W. (1986) A model for receptor-regulated calcium entry. Cell Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Putney J.W. (1990) Capacitative calcium entry revisited. Cell Calcium, 11, 611–624. [DOI] [PubMed] [Google Scholar]
- Rudolph H.K., Antebi,A., Fink,G.R., Buckley,C.M., Dorman,T.E., LeVitre,J., Davidow,L.S., Mao,J.I. and Moir,D.T. (1989) The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell, 58, 133–145. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanders S.L., Whitfield,K.M., Vogel,J.P., Rose,M.D. and Schekman,R.W. (1992) Sec61p and BiP directly facilitate polypeptide trans location into the ER. Cell, 69, 353–365. [DOI] [PubMed] [Google Scholar]
- Scidmore M.A., Okamura,H.H. and Rose,M.D. (1993) Genetic inter actions between KAR2 and SEC63, encoding eukaryotic homologues of DnaK and DnaJ in the endoplasmic reticulum. Mol. Biol. Cell, 4, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Hicks,J.B. and Fink,G.R. (1986) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Shi Y., Vattem,K.M., Sood,R., An,J., Liang,J., Stramm,L. and Wek,R.C. (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol., 18, 7499–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C. and Walter,P. (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell, 90, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A.M. and Cyert,M.S. (1997) Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes Dev., 11, 3432–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayle J., Pozzan,T. and Rudolph,H.K. (1999) Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 µM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J., 18, 4733–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka Y., Nakagawa,Y., Sato,C., Mino,M., Uozumi,N., Murata,N., Muto,S. and Iida,H. (2000) yam8+, a Schizosaccharomyces pombe gene, is a potential homologue of the Saccharomyces cerevisiae MID1 gene encoding a stretch-activated Ca2+-permeable channel. Biochem. Biophys. Res. Commun., 269, 265–269. [DOI] [PubMed] [Google Scholar]
- Tirasophon W., Welihinda,A.A. and Kaufman,R.J. (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev., 12, 1812–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J.S. and Lampen,O. (1975) Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem. Biophys. Res. Commun., 65, 248–257. [DOI] [PubMed] [Google Scholar]
- Vogel J.P., Misra,L.M. and Rose,M.D. (1990) Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol., 110, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J.W., Chrebet,G., Brodsky,G., Rolfe,M. and Rothstein,R. (1989) A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell, 58, 409–419. [DOI] [PubMed] [Google Scholar]
- Wang X.Z., Harding,H.P., Zhang,Y., Jolicoeur,E.M., Kuroda,M. and Ron,D. (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J., 17, 5708–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo A.S. et al. (2000) Presenilin-mediated modulation of capacitative calcium entry. Neuron, 27, 561–572. [DOI] [PubMed] [Google Scholar]
- Zhao C., Jung,U.S., Garrett-Engele,P., Roe,T., Cyert,M.S. and Levin,D.E. (1998) Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol., 18, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]