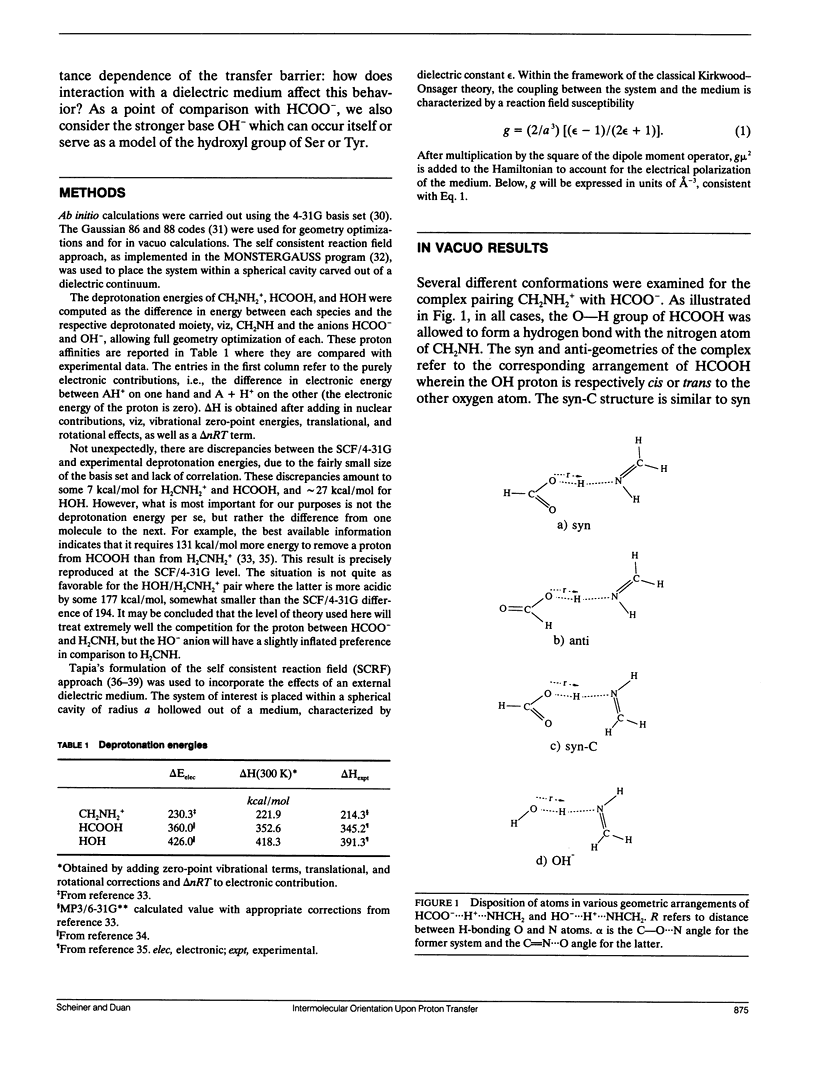
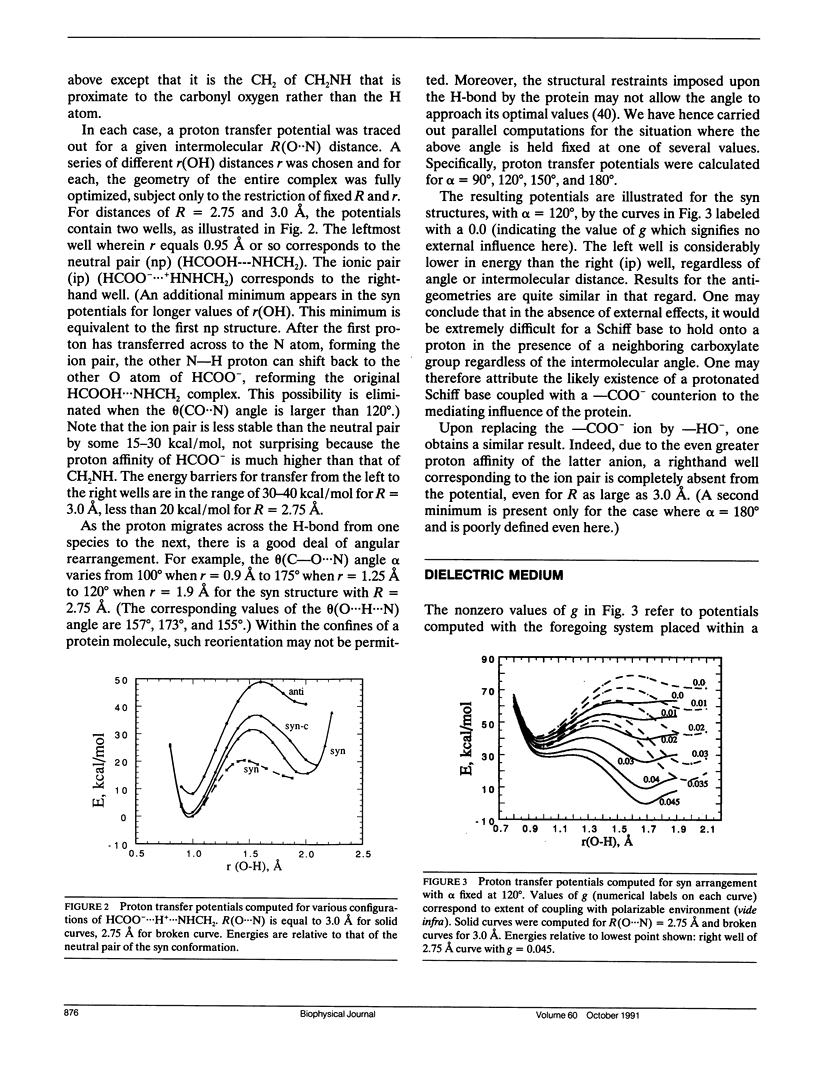
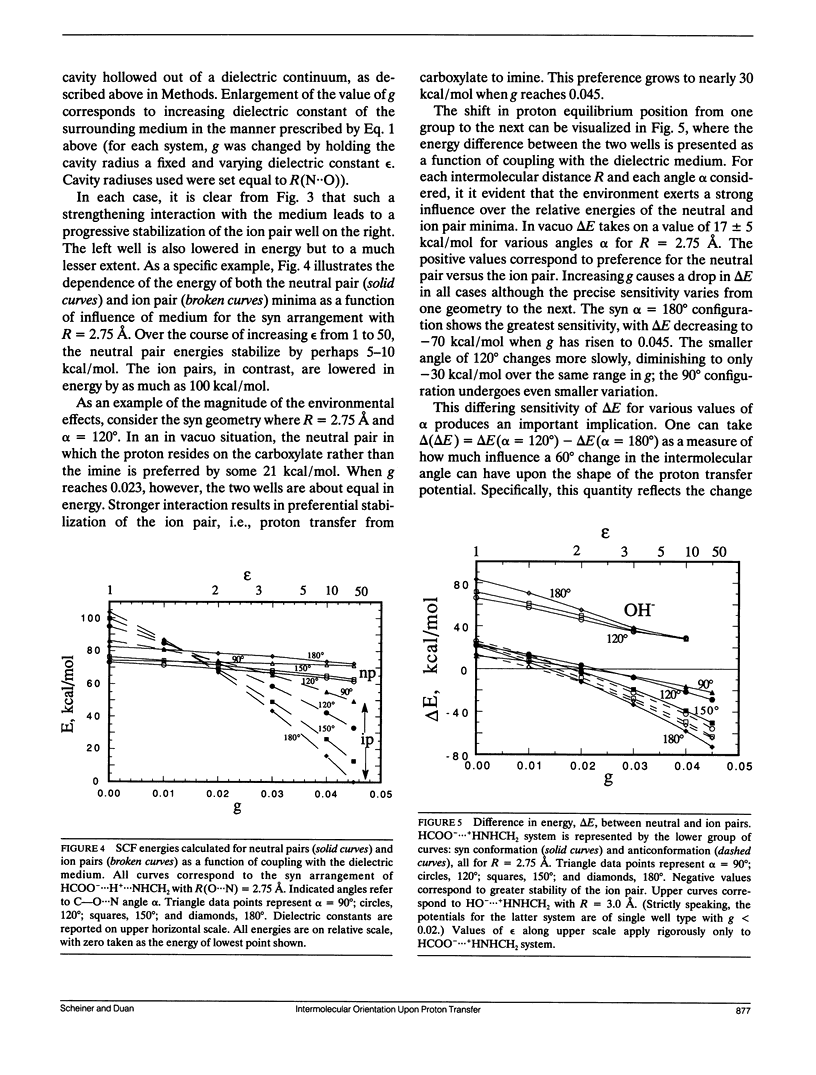
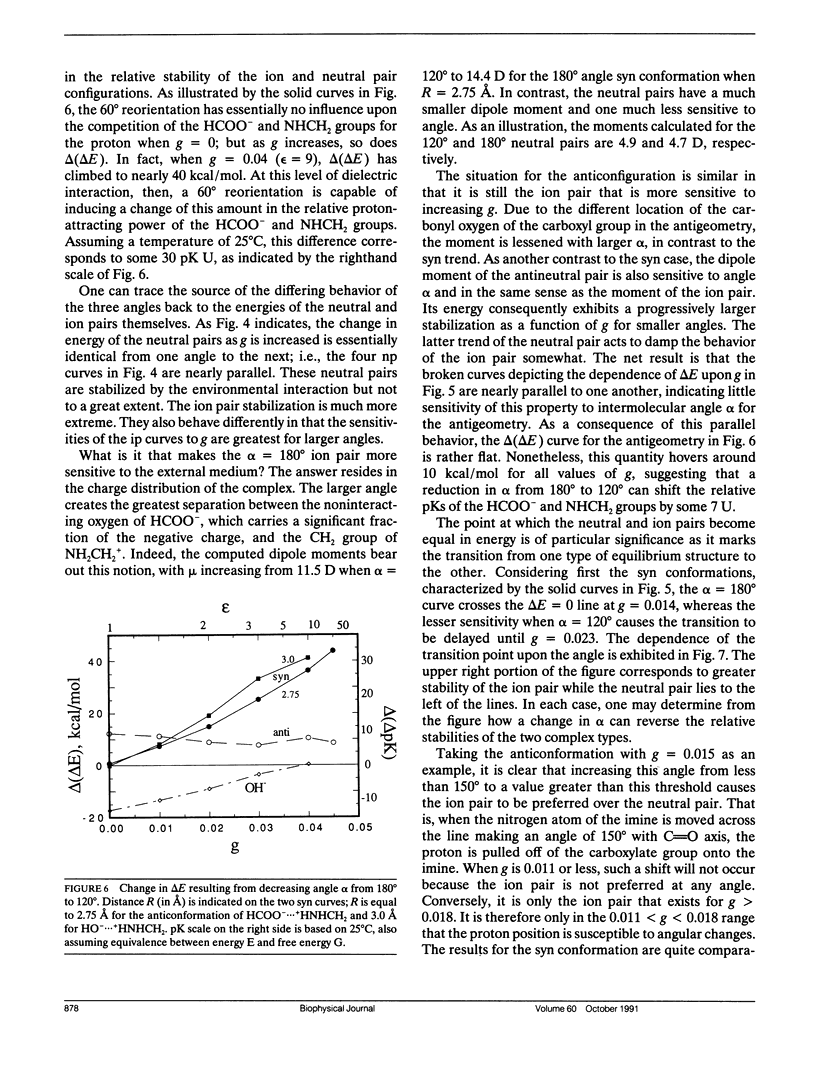
Abstract
Ab initio calculations are used to investigate the proton transfer process in bacteriorhodopsin. HN = CH2 serves as a small prototype of the Schiff base while HCOO- models its carboxylate-containing counterion and HO- the hydroxyl group of water of tyrosine, leading to the HCOO-..H+..NHCH2 and HO-..H+..NHCH2 complexes. In isolation, both complexes prefer a neutral pair configuration wherein the central proton is associated with the anion. However, the Schiff base may be protonated in the former complex, producing the HCOO-..+HNHCH2 ion pair, when there is a high degree of dielectric coupling with an external polarizable medium. Within a range of intermediate level coupling, the equilibrium position of the proton (on either the carboxylate or Schiff base) can be switched by suitable changes in the intermolecular angle. pK shift resulting from a 60 degrees reorientation are calculated to be some 5-12 pK U within the coupling range where proton transfers are possible. The energy barrier to proton transfer reinforces the ability of changes in angle and dielectric coupling to induce a proton transfer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N., Hubbard R. E. Hydrogen bonding in globular proteins. Prog Biophys Mol Biol. 1984;44(2):97–179. doi: 10.1016/0079-6107(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Cho Y. K., Cook P. F. pH dependence of the kinetic parameters for the pyrophosphate-dependent phosphofructokinase reaction supports a proton-shuttle mechanism. Biochemistry. 1989 May 16;28(10):4155–4160. doi: 10.1021/bi00436a005. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- Duñach M., Berkowitz S., Marti T., He Y. W., Subramaniam S., Khorana H. G., Rothschild K. J. Ultraviolet-visible transient spectroscopy of bacteriorhodopsin mutants. Evidence for two forms of tyrosine-185----phenylalanine. J Biol Chem. 1990 Oct 5;265(28):16978–16984. [PubMed] [Google Scholar]
- Gerwert K., Hess B., Soppa J., Oesterhelt D. Role of aspartate-96 in proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4943–4947. doi: 10.1073/pnas.86.13.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell E. E., Villafranca J. E., Warren M. S., Oatley S. J., Kraut J. Functional role of aspartic acid-27 in dihydrofolate reductase revealed by mutagenesis. Science. 1986 Mar 7;231(4742):1123–1128. doi: 10.1126/science.3511529. [DOI] [PubMed] [Google Scholar]
- Lin S. W., Mathies R. A. Orientation of the protonated retinal Schiff base group in bacteriorhodopsin from absorption linear dichroism. Biophys J. 1989 Oct;56(4):653–660. doi: 10.1016/S0006-3495(89)82712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz H., Zundel G. Thermodynamics of proton transfer in carboxylic acid-retinal Schiff base hydrogen bonds with large proton polarizability. Biochem Biophys Res Commun. 1986 Jul 31;138(2):819–825. doi: 10.1016/s0006-291x(86)80570-8. [DOI] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S., Hillenbrand E. A. Modification of pK values caused by change in H-bond geometry. Proc Natl Acad Sci U S A. 1985 May;82(9):2741–2745. doi: 10.1073/pnas.82.9.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheves M., Albeck A., Friedman N., Ottolenghi M. Controlling the pKa of the bacteriorhodopsin Schiff base by use of artificial retinal analogues. Proc Natl Acad Sci U S A. 1986 May;83(10):3262–3266. doi: 10.1073/pnas.83.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N., Vincent S. H. Proton transfer in the catalytic mechanism of carbonic anhydrase. CRC Crit Rev Biochem. 1983;14(3):207–255. doi: 10.3109/10409238309102794. [DOI] [PubMed] [Google Scholar]
- Tapia O., Sussman F., Poulain E. Environmental effects on H-bond potentials: A SCRF MO CNDO/2 study of some model systems. J Theor Biol. 1978 Mar 7;71(1):49–72. doi: 10.1016/0022-5193(78)90213-8. [DOI] [PubMed] [Google Scholar]
- Thole B. T., Van Duijnen P. T. Reaction field effects on proton transfer in the active site of actinidin. Biophys Chem. 1983 Jul;18(1):53–59. doi: 10.1016/0301-4622(83)80026-x. [DOI] [PubMed] [Google Scholar]


