Abstract
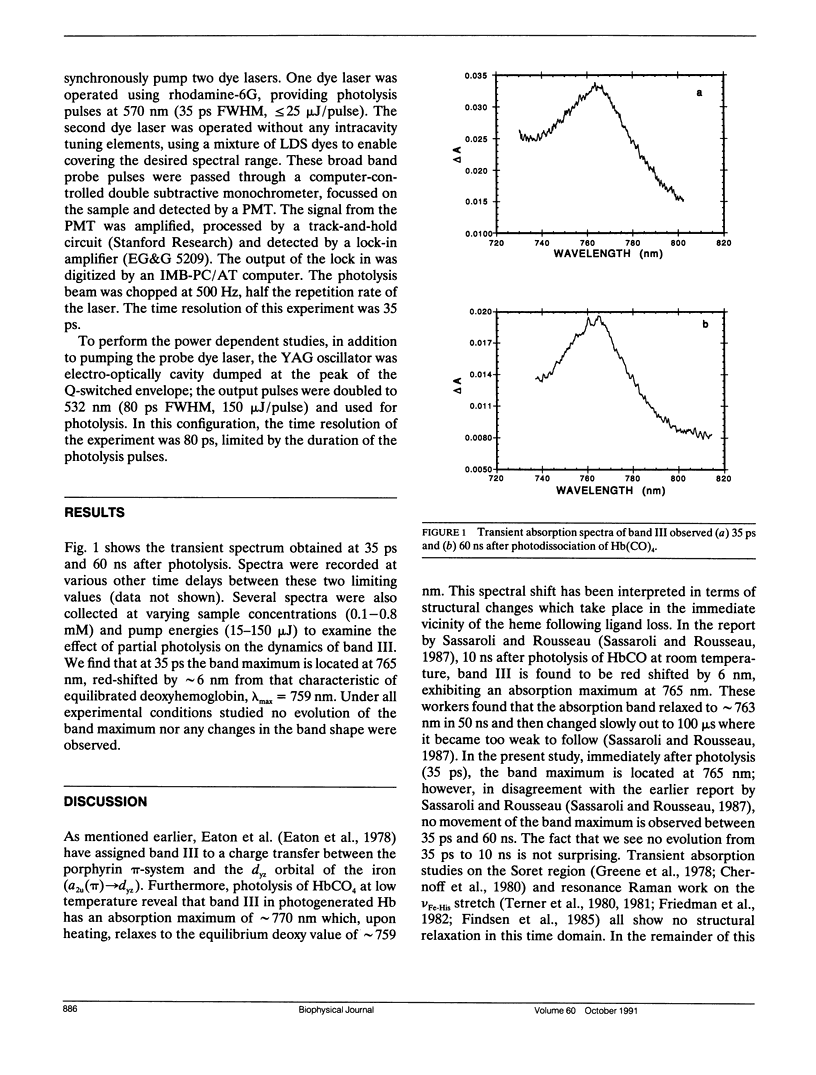
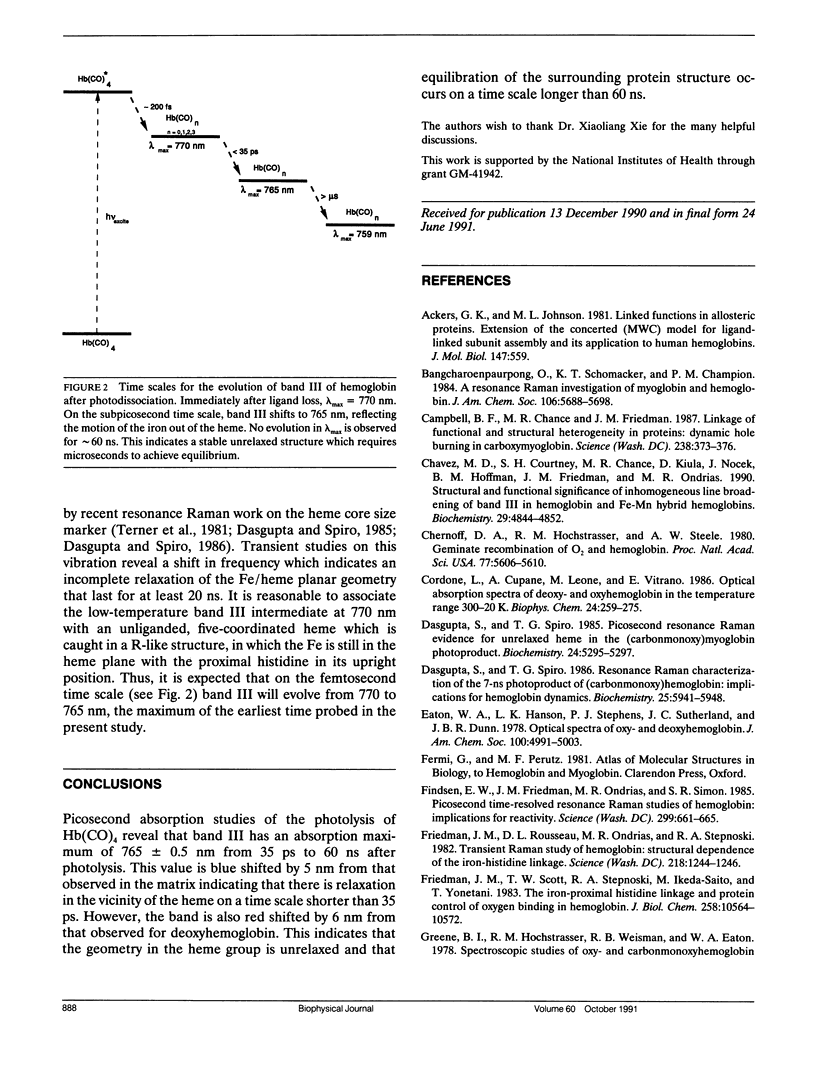
Picosecond absorption spectroscopy is used to examine the position and band shape of the near infrared absorption band of hemoglobin as a function of time after the photodissociation of CO from carbonmonoxyhemoglobin. For the earliest delay time probed, 35 ps, the peak of the transient spectrum is at 765 nm, red shifted by 6 nm from that characteristic of equilibrium deoxyhemoglobin. No evolution in either the peak position or band shape is observed for time delays up to 60 ns. In addition, the position and shape of the spectrum are independent of photolysis energies ranging from 15 microJ/pulse to 150 microJ/pulse, spanning conditions under which the photon/heme ratio is varied from 0.01 to 2.0. This indicates that the geometry in the heme group is unrelaxed and that equilibration of the surrounding protein structure occurs on a time scale longer than 60 ns.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Johnson M. L. Linked functions in allosteric proteins. Extension of the concerted (MWC) model for ligand-linked subunit assembly and its application to human hemoglobins. J Mol Biol. 1981 Apr 25;147(4):559–582. doi: 10.1016/0022-2836(81)90400-9. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Linkage of functional and structural heterogeneity in proteins: dynamic hole burning in carboxymyoglobin. Science. 1987 Oct 16;238(4825):373–376. doi: 10.1126/science.3659921. [DOI] [PubMed] [Google Scholar]
- Chavez M. D., Courtney S. H., Chance M. R., Kiula D., Nocek J., Hoffman B. M., Friedman J. M., Ondrias M. R. Structural and functional significance of inhomogeneous line broadening of band III in hemoglobin and Fe-Mn hybrid hemoglobins. Biochemistry. 1990 May 22;29(20):4844–4852. doi: 10.1021/bi00472a014. [DOI] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordone L., Cupane A., Leone M., Vitrano E. Optical absorption spectra of deoxy- and oxyhemoglobin in the temperature range 300-20 K. Relation with protein dynamics. Biophys Chem. 1986 Aug;24(3):259–275. doi: 10.1016/0301-4622(86)85031-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Spiro T. G., Johnson C. K., Dalickas G. A., Hochstrasser R. M. Picosecond resonance Raman evidence for unrelaxed heme in the (carbonmonoxy)myoglobin photoproduct. Biochemistry. 1985 Sep 24;24(20):5295–5297. doi: 10.1021/bi00341a003. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Spiro T. G. Resonance Raman characterization of the 7-ns photoproduct of (carbonmonoxy)hemoglobin: implications for hemoglobin dynamics. Biochemistry. 1986 Oct 7;25(20):5941–5948. doi: 10.1021/bi00368a016. [DOI] [PubMed] [Google Scholar]
- Findsen E. W., Friedman J. M., Ondrias M. R., Simon S. R. Picosecond time-resolved resonance Raman studies of hemoglobin: implications for reactivity. Science. 1985 Aug 16;229(4714):661–665. doi: 10.1126/science.4023704. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Rousseau D. L., Ondrias M. R., Stepnoski R. A. Transient Raman study of hemoglobin: structural dependence of the iron-histidine linkage. Science. 1982 Dec 17;218(4578):1244–1246. doi: 10.1126/science.7146910. [DOI] [PubMed] [Google Scholar]
- Friedman J. M., Scott T. W., Stepnoski R. A., Ikeda-Saito M., Yonetani T. The iron-proximal histidine linkage and protein control of oxygen binding in hemoglobin. A transient Raman study. J Biol Chem. 1983 Sep 10;258(17):10564–10572. [PubMed] [Google Scholar]
- Greene B. I., Hochstrasser R. M., Weisman R. B., Eaton W. A. Spectroscopic studies of oxy- and carbonmonoxyhemoglobin after pulsed optical excitation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R., Levitt M., Eaton W. A. Molecular dynamics simulation of photodissociation of carbon monoxide from hemoglobin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2034–2038. doi: 10.1073/pnas.82.7.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T., Yamamoto H., Kotani M., Yonetani T. Low temperature photodissociation of hemoproteins: carbon monoxide complex of myoglobin and hemoglobin. Biochim Biophys Acta. 1974 Nov 5;371(1):126–139. doi: 10.1016/0005-2795(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Leone M., Cupane A., Vitrano E., Cordone L. Dynamic properties of oxy- and carbonmonoxyhemoglobin probed by optical spectroscopy in the temperature range of 300-20 K. Biopolymers. 1987 Oct;26(10):1769–1779. doi: 10.1002/bip.360261009. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Sassaroli M., Rousseau D. L. Time dependence of near-infrared spectra of photodissociated hemoglobin and myoglobin. Biochemistry. 1987 Jun 2;26(11):3092–3098. doi: 10.1021/bi00385a022. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Smulevich G., Su C. Probing protein structure and dynamics with resonance Raman spectroscopy: cytochrome c peroxidase and hemoglobin. Biochemistry. 1990 May 15;29(19):4497–4508. doi: 10.1021/bi00471a001. [DOI] [PubMed] [Google Scholar]
- Terner J., Stong J. D., Spiro T. G., Nagumo M., Nicol M., El-Sayed M. A. Picosecond resonance Raman spectroscopic evidence for excited-state spin conversion in carbonmonoxy-hemoglobin photolysis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1313–1317. doi: 10.1073/pnas.78.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Energy-structure correlation in metalloporphyrins and the control of oxygen binding by hemoglobin. Proc Natl Acad Sci U S A. 1977 May;74(5):1789–1793. doi: 10.1073/pnas.74.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X. L., Simon J. D. Protein conformational relaxation following photodissociation of CO from carbonmonoxymyoglobin: picosecond circular dichroism and absorption studies. Biochemistry. 1991 Apr 16;30(15):3682–3692. doi: 10.1021/bi00229a013. [DOI] [PubMed] [Google Scholar]


