Abstract
Overexpression of ErbB-2/HER2 is associated with aggressive human malignancies, and therapeutic strategies targeting the oncoprotein are currently in different stages of clinical application. Tyrosine kinase inhibitors (TKIs) that block the nucleotide-binding site of the kinase are especially effective against tumors. Here we report an unexpected activity of TKIs: along with inhibition of tyrosine phosphorylation, they enhance ubiquitylation and accelerate endocytosis and subsequent intracellular destruction of ErbB-2 molecules. Especially potent is an irreversible TKI (CI-1033) that alkylates a cysteine specific to ErbB receptors. The degradative pathway stimulated by TKIs appears to be chaperone mediated, and is common to the heat shock protein 90 (Hsp90) antagonist geldanamycin and a stress-induced mechanism. In agreement with this conclusion, CI-1033 and geldanamycin additively inhibit tumor cell growth. Based upon a model for drug-induced degradation of ErbB-2, we propose a general strategy for selective destruction of oncoproteins by targeting their interaction with molecular chaperones.
Keywords: geldanamycin/growth factor/protein kinase inhibitors/stress response/ubiquitin
Introduction
The ErbB/HER subgroup of receptor tyrosine kinases includes four receptors with distinct recognition specificities to ligands of the epidermal growth factor (EGF) family. These ligands and receptors constitute a signaling network in which ErbB-2 acts as a ligand-less receptor that amplifies growth factor signaling (Yarden and Sliwkowski, 2001). ErbB receptors, as well as their ligands, are frequently involved in human cancer: autocrine synthesis of specific ligands and co-expression of a cognate receptor characterize tumors of epithelial and other origins. Amplification and consequent overexpression of the ErbB-2 gene (also called neu and HER2) characterize a relatively aggressive subset of breast and ovarian tumors (Slamon et al., 1989). The association of ErbB-2 overexpression with poor prognosis of several types of carcinomas led to recognition of the therapeutic potential of drugs that target the oncoprotein (reviewed in Yarden and Sliwkowski, 2001).
One therapeutic approach that has already reached clinical application is the use of an unarmed monoclonal antibody (mAb) called Trastuzumab (Herceptin™) (Pegram and Slamon, 2000). Studies performed in vitro and in mice have attributed the therapeutic potential of anti-ErbB-2 antibodies to their ability to enhance intracellular degradation of the cell surface-localized oncoprotein (Kasprzyk et al., 1992). An alternative, though significantly less specific, way to enhance intracellular degradation of ErbB-2 involves targeting of the heat shock protein 90 (Hsp90) by using benzoquinone ansamycins such as geldanamycin (GA) (Zheng et al., 2000; Xu et al., 2001a). Hsp90 forms complexes with ErbB-2 (Xu et al., 2001a) and other client proteins (reviewed in Buchner, 1999). Once GA blocks ATP binding to Hsp90, the chaperone complex associated with the client protein is biased towards a degradative fate, resulting in poly-ubiquitylation and subsequent destruction of the client (Neckers et al., 1999). The therapeutic safety and efficacy of GA derivatives and other antagonists of Hsp90 are currently being tested in clinical trials. However, their potentially broad effect due to the multiplicity of Hsp90-binding client proteins is a matter of concern. In contrast, another group of drugs, which are in advanced stages of clinical testing, block the nucleotide-binding site of ErbB proteins rather than the respective site of Hsp90 (Levitzki, 1999; Fry, 2000). These tyrosine kinase inhibitors (TKIs) offer very high selectivity to specific nucleotide-binding sites. Consequent to blocking kinase activity, most downstream signaling pathways are inhibited, which leads to growth arrest of tumors whose proliferation depends on ErbB signaling. A new generation of TKIs is designed to alkylate a prominent cysteine residue uniquely positioned in the nucleotide-binding pocket of ErbB-1 and ErbB-2, thus allowing irreversible kinase inhibition (Fry, 2000). A series of such compounds has been shown to inhibit tumor growth in animals more effectively than the corresponding reversible TKIs (Fry et al., 1998).
Strategies combining the effectiveness of chaperone-mediated degradation with the selectivity of TKIs hold promise for cancer therapy, but they are currently unavailable. Our present study was initiated by an observation that mutagenesis of the kinase domain of ErbB-1 sensitizes the receptor to GA. Because recent results suggest that ErbB-2 is an excellent target for a GA-inducible pathway (Tikhomirov and Carpenter, 2000; Xu et al., 2001a), this observation raised the possibility that blocking the nucleotide-binding site of ErbB-2 with a kinase inhibitor will bias the chaperone-mediated system to a degradative fate for the oncoprotein. Here, we present results that biochemically support this mechanism and offer a strategy to selectively target chaperoned oncogene products to effective destruction.
Results
A kinase-defective mutant of ErbB-1 is a preferred substrate for the GA-induced mechanism of receptor ubiquitylation
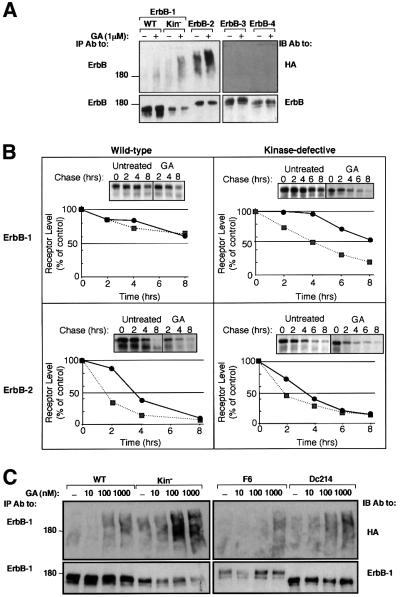
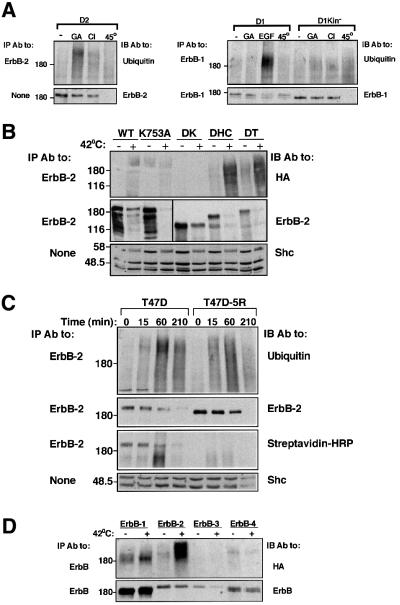
The sensitivity of ErbB proteins to treatment with GA was analyzed in Chinese hamster ovary cells (CHO; Figure 1A). Whereas ErbB-1 underwent faint ubiquitylation upon treatment of cells with GA, the drug significantly increased poly-ubiquitylation of ErbB-2, but no modification of ErbB-3 or ErbB-4 was detectable. Interestingly, a kinase-defective mutant of ErbB-1 (in which Lys721 of the nucleotide-binding domain has been replaced by an alanine) displayed enhanced basal and GA-induced ubiquitylation relative to the native receptor, and its stability was reduced by the drug (>40% reduction). The sensitivity to GA was further tested by using metabolic labeling of ErbB-1, ErbB-2 and their respective kinase-defective mutants (Figure 1B). Evidently, the half-life of ErbB-1 (∼10 h) is longer than that of ErbB-2 and its kinase-defective form (∼3.5 h), but unlike the GA-sensitive ErbB-2 [half-life: wild type (WT), ∼1.5 h; kinase defective, ∼2 h], ErbB-1 was barely affected by the drug. In contrast, GA enhanced the decay rate of the kinase-defective form of ErbB-1, reducing its half-life to ∼4 h. The relatively high GA sensitivity of the kinase-defective ErbB-1 mutant was not due to defective autophosphorylation, because mutants whose autophosphorylation is severely reduced due to either deletion of most of the C-terminus (mutant denoted Dc214), or to replacement of six major tyrosine autophosphorylation sites with phenylalanine residues (mutant denoted F6), exhibited sensitivity similar to that of the wild-type receptor (Figure 1C). In conclusion, GA-induced ubiquitylation and degradation of ErbB-1 and ErbB-2 are specifically enhanced by perturbation of the nucleotide-binding pocket of the receptor tyrosine kinase.
Fig. 1. Differential sensitivity of ErbB proteins to geldanamycin. (A) The indicated ErbB receptors, including a kinase-defective ErbB-1 (Kin–), were transiently expressed in CHO cells, along with a vector driving expression of HA-Ub. Forty-eight hours post-transfection, cells were treated with GA (1 µM) for 1 h at 37°C (+) or left untreated (–). Cell extracts were then prepared and mAbs specific to the respective ErbB protein were used for immunoprecipitation (IP). Immuno precipitated proteins were electrophoretically separated and transferred to nitrocellulose membranes. Ubiquitylated proteins were detected by immunoblotting (IB) with an anti-HA antibody. Membranes were subsequently stripped and re-blotted with receptor-specific polyclonal antibodies. The location of a molecular size marker is indicated in kilodaltons. (B) The rates of turnover of ErbB-1 and ErbB-2, as well as their respective kinase-defective mutants, were determined by transfecting Cos-7 cells with the corresponding expression vectors. Thirty-six hours later, cells were subjected to metabolic labeling with 35S-labeled amino acids for 12 h. Following washes, cells were treated in media containing no radioactivity, in the absence (circles) or presence (squares) of 1 µM GA. Following the indicated time intervals, cells were extracted and the receptors immunoprecipitated. Autoradiograms (insets) and the corresponding decay curves are shown. (C) The indicated ErbB-1 proteins were co-expressed in CHO cells along with HA-Ub. The following mutants of ErbB-1 were used, along with the wild-type (WT) form: a kinase-defective mutant (K721A; Kin–), a receptor defective in six major tyrosine autophosphorylation sites (F6) and a deletion mutant lacking 214 C-terminal amino acids (Dc214). Cells were treated at 37°C for 2 h with the indicated increasing concentrations of GA, and extracts analyzed as in (A). All experiments presented in this and other figures were repeated at least three times.
An irreversible kinase inhibitor targets ErbB-2 to internalization, ubiquitylation and proteasomal degradation
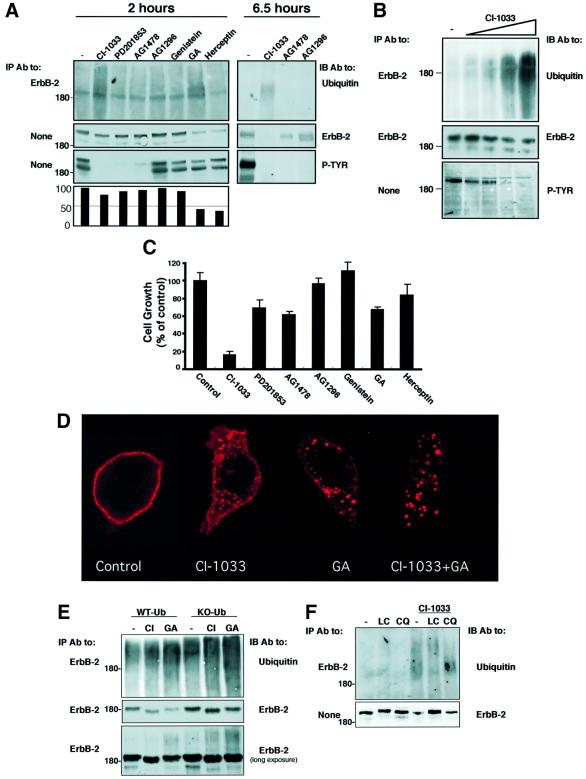
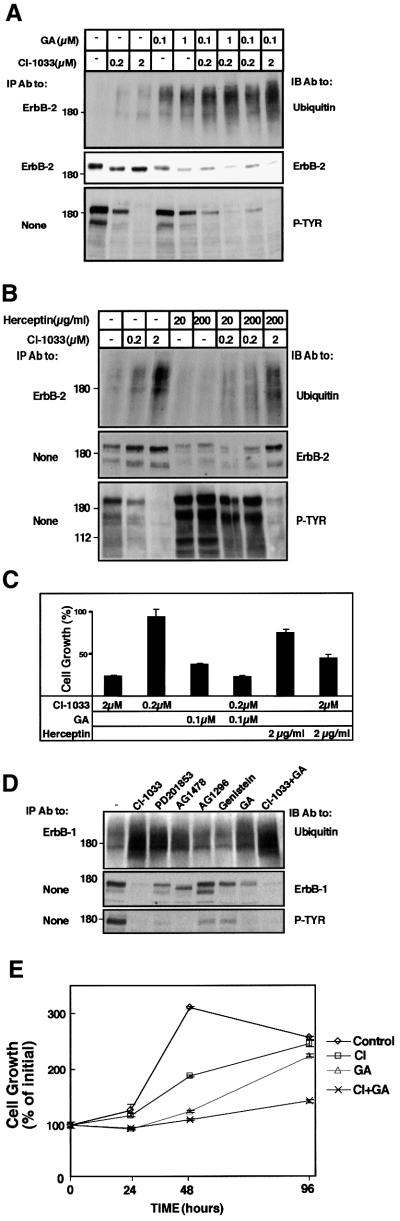
The observation that a kinase-dead ErbB-1 is a preferred substrate for the GA-induced pathway predicted that the effect of the kinase-defecting mutation might be mimicked by drugs specific to the nucleotide-binding site of ErbB proteins. To test this prediction on ErbB-2, we selected N87 human gastric carcinoma cells, because tumorigenic growth of these cells is driven by the oncoprotein and can be inhibited by specific mAbs (Kasprzyk et al., 1992; Klapper et al., 1997). Cells were exposed to a set of TKIs (at 10 µM), which included three ErbB-specific inhibitors: AG1478 (Gazit et al., 1996); CI-1033, a highly potent irreversible inhibitor; and its reversible analog PD201853 (Smaill et al., 2001). Examination of tyrosine phosphorylation in whole-cell extracts confirmed the reported efficacy of the various inhibitors (Figure 2A), and also verified that ErbB-2 is the most abundant tyrosine phosphorylated protein in N87 cells. Addressing the effect on receptor degradation, we found that GA and Herceptin significantly reduced receptor levels following 2 h of treatment, and CI-1033 partly reduced ErbB-2 expression at this time, while other kinase inhibitors were less effective. Consistent with these observations, the effects of both CI-1033 and GA were associated with enhanced ubiquitylation of ErbB-2 (Figure 2A). As expected, the degradative action of CI-1033 was more pronounced after a longer incubation (6.5 h), but PD201853 and AG-1478 displayed reduced effects. The degradation/ubiquitylation effect of CI-1033 was confirmed when increasing concentrations of the drug were tested on N87 cells (Figure 2B). The effect of CI-1033 was additionally addressed utilizing metabolic labeling experiments (as in Figure 1B), in which CI-1033 reduced the half-life of ErbB-2 to 1.5 h, but produced no effect on ErbB-1 (data not shown). In an attempt to correlate the degradative effects with inhibition of ErbB-2-dependent tumor cell growth, we tested cell proliferation in the presence of the various drugs (Figure 2C). In this assay, both Herceptin and GA were inhibitory, but the non-ErbB-specific TKIs genistein and AG-1296 were ineffective. By contrast, all three ErbB-specific TKIs effectively reduced cell proliferation, with the irreversible inhibitor achieving complete arrest of growth. These observations extend previous reports on a linkage between inhibition of ErbB phosphorylation and cell growth (Vincent et al., 2000), and suggest that drug-induced receptor degradation contributes to the cytostatic effect of TKIs.
Fig. 2. A specific irreversible tyrosine kinase inhibitor enhances internalization, ubiquitylation and proteasomal degradation of ErbB-2, and inhibits growth of ErbB-2-overexpressing tumor cells. (A) N87 gastric cancer cells were treated for either 2 or 6.5 h with the indicated TKIs, GA (1 µM) and Herceptin (20 µg/ml). The short treatment with TKIs (10 µM each) was carried out in serum-free medium, but longer incubations were performed in serum-containing medium and higher TKI concentrations (20 µM). Cell extracts were prepared and either directly resolved by electrophoresis (panels labeled None) or they were first subjected to IP, followed by IB with the indicated antibodies (P-TYR; phosphotyrosine). The lower panel shows quantification of the corresponding ErbB-2 protein bands as percentages of control. (B) N87 cells were treated for 2.5 h with increasing concentrations of CI-1033 (20 nM, 200 nM, 2 µM and 20 µM) or solvent only (DMSO; lane –). IP and IB were performed with the indicated antibodies. (C) N87 cells were incubated with the indicated kinase inhibitors (each at 10 µM), GA (1 µM) or Herceptin (20 µg/ml). Control cells were incubated with DMSO. The extent of cell proliferation was determined following 48 h of incubation by using the MTT assay. Averages of six determinations are presented along with their standard deviation (SD) values. (D) N87 cells growing on glass slides were treated for 4 h with CI-1033 (10 µM), GA (1 µM) or a mixture of both compounds. Cells were then fixed, permeabilized and ErbB-2 detected by using an anti-ErbB-2 antibody and a fluorescently labeled secondary antibody to murine immunoglobulins. Representative confocal microscopy images through middle sections of treated cells are presented. (E) HEK-293T cells were transfected with vectors encoding ErbB-2 and either wild-type ubiquitin or a lysine-less mutant of ubiquitin (KO-Ub). Sixteen hours later, cells were treated for 2.5 h with CI-1033 (10 µM) or GA (1 µM), and ErbB-2 protein was immunoprecipitated and analyzed by IB as indicated. Note the relatively small fraction of ubiquitylated ErbB-2 (lower panel). (F) N87 cells were treated with chloroquine (CQ; 0.1 mM) or lactacystin (LC; 10 µM) for 40 min, followed by treatment with CI-1033 (10 µM). Following cell lysis, equal amounts of extracts were subjected to IB, either directly or after IP, as indicated.
Next, we tested the prediction that CI-1033, like GA (Mimnaugh et al., 1996; Tikhomirov and Carpenter, 2000), can induce internalization of surface ErbB-2 prior to intracellular degradation. Staining for ErbB-2 in untreated N87 cells demonstrated that the receptor is localized primarily to the plasma membrane, but treatment with either CI-1033 or GA induced aggregation of ErbB-2 molecules into large, sub-membranal clusters, which may represent endosomes (Figure 2D). Subsequent experiments suggested that drug-induced endocytosis targets ErbB-2 molecules to proteasomal, rather than lysosomal, degradation. Thus, by using a lysine-less ubiquitin mutant (KO-Ub), which upon conjugation inhibits ubiquitin chain elongation, a prerequisite for proteasomal degradation (Thrower et al., 2000), we learned that ErbB-2 degradation induced by CI-1033 and GA depends on receptor poly-ubiquitylation (Figure 2E). Consistent with degradation by the proteasome, chloroquine, a weak base that alkalinizes the lysosome, was ineffective, but lactacystin, an antagonist of proteasomal proteinases, inhibited ErbB-2 degradation upon treatment of cells with CI-1033 (Figure 2F). This observation is in line with published data relating to the proteasomal degradation of ErbB-2 in response to GA (Mimnaugh et al., 1996). In conclusion, an irreversible TKI, which is a highly potent growth inhibitor for ErbB-2-driven tumor cells, enhances endocytosis, ubiquitylation and subsequent proteasomal degradation of the oncogenic receptor.
CI-1033 and GA act on both mature and nascent ErbB-2 molecules
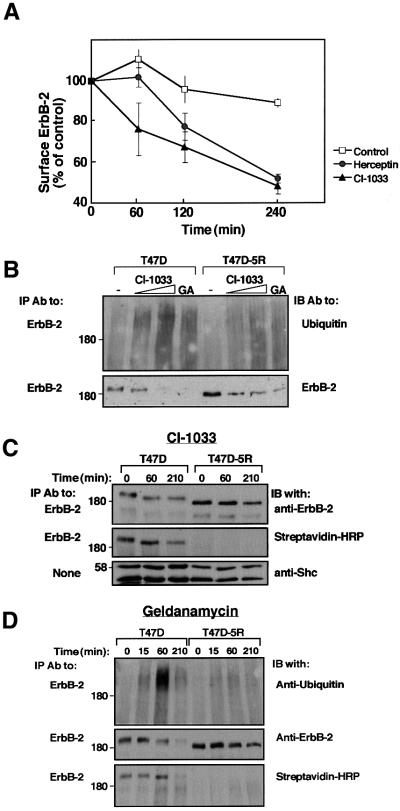
To address the cellular site of action of CI-1033, we employed ErbB-2-overexpressing T47D breast cancer cells and their derivative, T47D-5R cells. In these cells, no ErbB-2 reaches the cell surface because an endoplasmic reticulum (ER)-resident single-chain antibody effectively retains the receptor in the ER (Beerli et al., 1994). First, we utilized a downregulation assay to confirm the capacity of CI-1033 to remove mature ErbB-2 from the surface of parental T47D cells (Figure 3A). The observed effect of CI-1033 on these cells was comparable in extent and kinetics to the action of a tumor-inhibitory antibody. Exposure of T47D-5R cells to CI-1033 increased ubiquitylation and accelerated degradation of the nascent form of ErbB-2, whose electrophoretic mobility is characteristically faster (Figure 3B). Comparison with the effects of CI-1033 on the mature form of ErbB-2 suggested that the ER-retained receptor is less accessible to the mechanism activated by the drug. It is noteworthy that experiments performed with other cell types concluded that the nascent, as well as the mature form of ErbB proteins are targeted by benzoquinone ansamycins (Murakami et al., 1994; Chavany et al., 1996; Xu et al., 2001b). Indeed, when applied on T47D cells, GA affected both forms of ErbB-2 (Figure 3B). Receptor degradation induced by each of the compounds was tested further in time-resolved experiments utilizing cell surface biotinylation (Figure 3C and D). The results corroborated in an independent way the ability of a TKI to enhance degradation of the surface-localized biotinylated ErbB-2. This analysis also verified lack of surface expression of ErbB-2 in the T47D-5R cell line, and when extended to GA, revealed a stronger effect on surface ErbB-2 relative to the ER-retained receptor.
Fig. 3. CI-1033 and GA enhance destruction of both mature and immature ErbB-2 molecules. (A) Monolayers of T47D cells were incubated at 37°C for the indicated time intervals in the presence of either CI-1033 (10 µM; triangles) or Herceptin (10 µg/ml; circles). Control monolayers were incubated in the absence of agents (squares). Levels of cell surface ErbB-2 were determined by incubation with a radiolabeled antibody. Each point represents the average ± SD of duplicates. (B) T47D or T47D-5R cells were treated for 3 h with increasing concentrations of CI-1033 (2 or 20 µM) or with GA (1 µM). Cell extracts were subjected to IP and IB with the indicated antibodies. Note the faster mobility of the nascent ErbB-2 of T47D-5R cells. (C) Cells were treated with CI-1033 (5 µM) for the indicated time intervals, followed by surface labeling for 40 min on ice with Biotin-X-NHS. Streptavidin–HRP denotes incubation of the membrane with streptavidin conjugated to peroxidase. (D) T47D or T47D-5R cells were treated with GA (1 µM) for the indicated time intervals, followed by surface labeling for 40 min on ice with Biotin-X-NHS. Cell extracts were analyzed with the indicated antibodies.
The catalytic domain of ErbB-2 is essential for drug-induced ubiquitylation and degradation
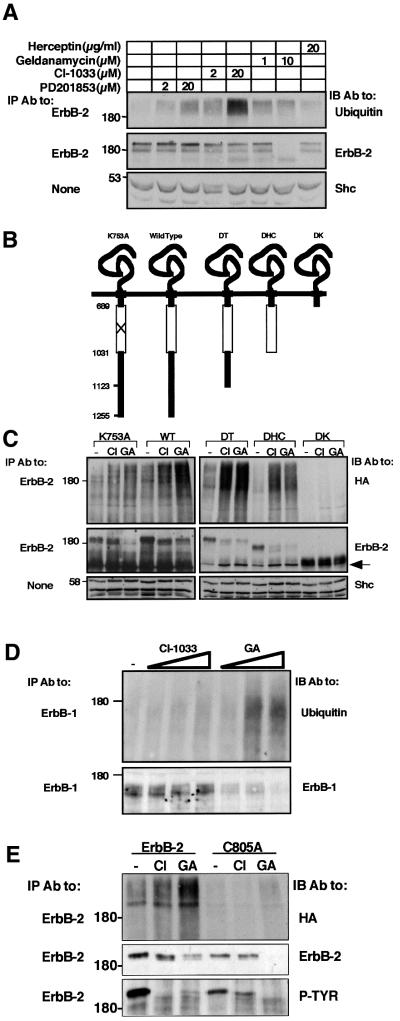
Affinity-labeling experiments indicated that CI-1033 is highly specific to ErbB proteins (Fry et al., 1998). Because these receptors heterodimerize extensively, we tested the ability of CI-1033 to target ErbB-2 to ubiquitylation in a null cellular background, free of effects due to receptor heterodimerization. To this end, we made use of 32D mouse myeloid cells, which are devoid of endogenous ErbBs (Pinkas-Kramarski et al., 1996). Treatment of ErbB-2-expressing 32D cells (D2 cells) with GA induced both ubiquitylation and degradation of the receptor, as did CI-1033, albeit inducing relatively enhanced ubiquitylation and comparatively delayed degradation (Figure 4A). By contrast, the reversible analog of CI-1033 elicited lower ubiquitylation of ErbB-2, and no evident degradation following 2 h of incubation. Interestingly, drug-induced ubiquitylation and degradation of ErbB-1 in 32D cells (D1 cells) was apparent only after relatively long incubation periods (data not shown). These observations implied that CI-1033 acts directly on each receptor, with a preference for ErbB-2. To map the susceptibility-conferring domain of ErbB-2, we made use of a previously described series of ErbB-2 deletion mutants transiently expressed in CHO cells (Figure 4B) (Xu et al., 2001a). Treatment with CI-1033 and GA led to ubiquitylation and degradation of ErbB-2 mutants lacking the C-terminal 132 (mutant denoted DT) or 224 (DHC) amino acids, but no effect was seen on a receptor lacking the whole C-terminus, including the kinase domain (DK; Figure 4C). Metabolic labeling experiments (identical to those presented in Figure 1B) demonstrated that CI-1033 produced more limited effects on a kinase-defective ErbB-1 than did GA (reducing the half-life to ∼6 h in comparison to ∼4 h by GA), possibly due to reduced binding of the compound to the mutant ATP-binding site (Fry et al., 1998). CI-1033 covalently binds Cys773 of ErbB-1 (Fry et al., 1998). Mutagenesis of the analogous residue of ErbB-2 (mutant denoted C805A) abolished sensitivity to CI-1033 and allowed recovery of ErbB-2 self-phosphorylation following removal of the drug (Figure 4E). Like in the case of kinase-defective ErbB-1 (Figures 4D and 1) and ErbB-2 (K753A; Figures 4C and 1A), C805A displayed enhanced sensitivity to GA. Taken together, the data presented in Figure 4 ascribe to the kinase domain a crucial role in drug-induced targeting of receptors to degradation. However, whereas integrity of the nucleotide-binding site appears to be important for the response to CI-1033, perturbation of this pocket enhances sensitivity to GA.
Fig. 4. Receptor sensitivity to drug-induced ubiquitylation and degradation is conferred by the kinase domain. (A) Liquid cultures of D2 cells were spun and resuspended in medium containing the indicated agents. Following 2 h of incubation at 37°C, cells were re-spun and their lysates subjected to IP and IB with the indicated antibodies. For control, whole-cell extracts were subjected to IB with antibodies to Shc. (B) Schematic representation of ErbB-2 mutant proteins. The horizontal line represents the plasma membrane and numbers indicate the amino acid sites of truncations. The open box marks the tyrosine kinase domain. (C) The indicated ErbB-2 constructs were expressed in CHO cells along with HA-Ub. Forty-eight hours after transfection, cells were treated with CI-1033 (CI; 5 µM), GA (1 µM) or DMSO (lanes labeled –) for 90 min. Cell extracts were prepared and analyzed with the indicated antibodies. IB for ErbB-2 was carried out using an antibody directed to the extracellular domain. An arrow marks a 135 kDa degradation product of ErbB-2. (D) 32D cells stably expressing a kinase-defective ErbB-1 mutant were treated for 3 h with increasing concentrations of CI-1033 (0.2, 2 or 20 µM) or GA (0.1, 1 or 10 µM). Thereafter, cell extracts were prepared and the ErbB-1 protein analyzed with the indicated antibodies. (E) HEK-293T cells were transfected with a plasmid encoding HA-Ub, along with expression vectors for either wild-type ErbB-2 or a mutant at Cys805 (C805A). Eighteen hours later, cells were treated for 20 min with CI-1033 (10 µM) or GA (1 µM), or left untreated for control. Thereafter, cells were washed and incubated for 4 h in fresh drug-free media prior to analysis of ErbB-2 with the indicated antibodies.
The stress-induced, chaperone-associated mechanism mediates degradation of CI-1033-treated ErbB-2
On the basis of the results obtained, we conjectured that the physiological correlate of the CI-1033-induced effects on ErbB-2 degradation would be reflected upon induction of stress. Indeed, exposure of 32D cells to heat shock demonstrated that this type of stress, like treatment with CI-1033 or GA, can target ErbB-2 and kinase-defective ErbB-1 to destruction, but it largely spares the wild-type form of ErbB-1 (Figure 5A). Likewise, transient expression of ErbB-2 deletion mutants in CHO cells revealed that the kinase domain is necessary for heat-induced ubiquitylation and degradation (Figure 5B; note some degradation of the DK mutant). In addition, by using T47D-5R cells, we found that thermal stress caused ubiquitylation and degradation of the mature form of ErbB-2, as well as the nascent species (Figure 5C). Similar to their pattern of sensitivities to GA (Figure 1A), the four ErbB proteins differed remarkably in their ubiquitylation upon heat shock (Figure 5D). Hence, the analogy between the effects of stress and those of CI-1033 suggests that TKIs target receptor tyrosine kinases to degradation by recruiting the stress-inducible machinery, a possibility consistent with the similar, yet distinct effects of GA, whose major target is Hsp90.
Fig. 5. The effect of thermal stress on ErbB proteins shares biochemical characteristics with the effects of CI-1033 and GA. (A) The indicated derivatives of 32D cells were treated for 1 h at 37°C with EGF (100 ng/ml), GA (1 µM) or CI-1033 (CI; 10 µM). Alternatively, cells were subjected to heat shock at the indicated temperature for 1 h or left untreated (lanes labeled –). Cell extracts were analyzed by IB and/or IP with the indicated antibodies. (B) The indicated ErbB-2 constructs were expressed in CHO cells along with HA-Ub. Forty-eight hours after transfection, cells were subjected to heat shock at 42°C for 60 min. Cell lysates were analyzed with the indicated antibodies. (C) T47D or T47D-5R cells were subjected to heat shock at 44°C for the indicated time intervals, followed by surface labeling for 40 min on ice with Biotin-X-NHS. Cell extracts were subjected to IP and IB with the indicated antibodies, or with a peroxidase-conjugated streptavidin. Note the reduction in Shc levels in the rightmost lane. (D) COS cells were transfected with expression vectors encoding HA-Ub and one of the four ErbB proteins. Forty-eight hours later, cells were subjected to heat stress for 60 min at 42°C as indicated (+) or left untreated (–). Following lysis, the respective ErbB proteins were analyzed with the indicated antibodies.
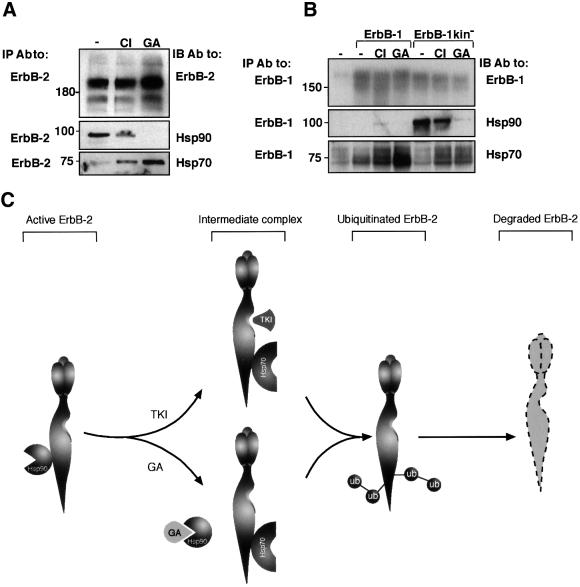
To address the molecular basis for the effect of CI-1033 on degradation of ErbB-2, we followed the interaction of the receptor with the molecular chaperones Hsp90 and Hsp70 upon exposure of N87 cells to CI-1033 or GA (Figure 6A). In accordance with published results (Xu et al., 2001a), GA caused dissociation of ErbB-2 from Hsp90, and enhanced the interaction of the receptor with Hsp70. Intriguingly, the effect of CI-1033 was similar but less pronounced: the drug slightly reduced the interaction with Hsp90, and enhanced binding to Hsp70. We next examined the interaction of the chaperones with ErbB-1 in COS cells (Figure 6B). As expected from the differential sensitivity of the kinase-defective receptor and the native receptor to GA (Figure 1A), ErbB-1 appeared uncoupled from Hsp90, whereas the kinase-defective mutant, which acquires GA sensitivity (Figure 1A), appeared to be strongly associated with the chaperone. Interestingly, treatment with CI-1033 slightly enhanced the interaction of ErbB-1 with Hsp90, perhaps by mimicking the effect of the kinase mutation. However, GA exerted no such effect, presumably because this drug directly inactivates Hsp90. Indeed, the kinase-defective mutant of ErbB-1 exhibited constitutively strong associations with Hsp90, but GA dissociated the complex more effectively than CI-1033. Unexpectedly, in spite of barely detectable binding to Hsp90, both drugs increased association of ErbB-1 with Hsp70. In essence, ErbB-2 is constitutively chaperoned by Hsp90 and this complex is disrupted by GA. CI-1033 appears to be a weaker antagonist of the interaction with the chaperone, but both drugs enhance associations with Hsp70.
Fig. 6. A chaperone complex including Hsp90 and Hsp70 is involved in drug-induced ubiquitylation and degradation of ErbB receptors. (A) N87 cells were treated for 1 h with CI-1033 (CI; 10 µM), GA (1 µM) or left untreated (–). Cells were extracted in TMNS buffer (containing low detergent concentration) and analyzed using the indicated antibodies. (B) COS cells were transfected with plasmids driving expression of either wild-type ErbB-1, a kinase-defective ErbB-1 (K721A; ErbB-1kin–) or left untreated (lanes labeled –), as indicated. Twenty-four hours after transfection, cells were treated for 1 h with CI-1033 (CI; 10 µM), GA (1 µM) or left untreated (–). Cells were then extracted in TMNS buffer, followed by IP and IB with antibodies to ErbB-1, Hsp90 and Hsp70, as indicated. Note the low level of ErbB-1 detected in untransfected COS cells. (C) Proposed model of drug-induced pathways leading to ubiquitylation and degradation of ErbB-2. ErbB-2 molecules are represented by a three-lobule extracellular domain and an elongated intracellular domain with a groove marking the ATP-binding site. According to the model, ErbB-2 is held at the plasma membrane in an active state through association with Hsp90. Dissociation of the complex is initiated by drug binding to the nucleotide-binding site of either ErbB-2 (e.g. CI-1033) or Hsp90 (e.g. GA). Subsequently, Hsp70 is recruited, ubiquitylation of ErbB-2 is promoted by an unknown mechanism, and followed by receptor degradation.
CI-1033 and GA act in an additive manner
Figure 6C summarizes our current model of drug-induced ubiquitylation and degradation of ErbB-2. According to this model, Hsp90 maintains ErbB-2 in a signaling-competent state at the plasma membrane. Both CI-1033 and GA can destabilize the complex with Hsp90, but they differ in their primary targets: whereas CI-1033 binds to the nucleotide-binding pocket of ErbB-2, GA blocks ATP binding to the nucleotide-binding site of Hsp90. Upon binding of either drug, an alternative complex comprising Hsp70 is favored, and subsequently the associated receptor undergoes ubiquitylation and degradation by still unknown machineries. Hence, TKIs and GA may direct receptor tyrosine kinases to the same degradation pathway, but they act upon different components of the chaperone– receptor complex. If correct, this model predicts that a combination of the drugs will additively enhance downregulation of ErbB-2 and ErbB-1 in human cancer cells.
In accordance with the working hypothesis, when applied alone on N87 tumor cells, CI-1033 induced only limited effects, but combinations with GA were significantly and reproducibly more effective (Figure 7A; compare the effects of 0.1 µM GA and 0.2 µM CI-1033 alone and in combination). In experiments that are not presented, we observed a small but reproducible additive effect of GA and a reversible TKI (PD201853). Likewise, an additive effect of CI-1033 and GA was seen on downregulation of surface ErbB-2 and induction of intracellular aggregates containing ErbB-2 (Figure 2D; data not shown). By contrast, combining CI-1033 with a mAb resulted in no additive effect on ErbB-2 degradation (Figure 7B). In fact, this combination was somewhat antagonistic. For example, combining Herceptin with a relatively high dose of CI-1033 (2 µM) significantly inhibited antibody-induced phosphorylation and degradation of ErbB-2, and concomitantly decreased drug-induced ubiquitylation of the receptor. To verify the relevance of drug combinations to inhibition of tumor cell growth, we treated N87 cells with drug combinations, and measured cell growth 4 days later (Figure 7C). Evidently, when presented alone, each drug exerted an inhibitory effect, and the combinations were in line with the effects we observed on ErbB-2 degradation: whereas the combination of CI-1033 (0.2 µM) and GA (0.1 µM) resulted in enhanced growth-inhibitory activity, a mixture of Herceptin (2 µg/ml) and CI-1033 (2 µM) was less effective than CI-1033 alone.
Fig. 7. CI-1033 and GA act additively in ubiquitylation and degradation of ErbB-2, and in inhibition of ErbB-dependent tumor cell growth. (A) N87 cells were treated for 3 h with the indicated concentrations of CI-1033, GA or a combination of the drugs. Cell lysates were subjected to the indicated analyses. (B) N87 cells were treated for 2.5 h with the indicated concentrations of CI-1033, Herceptin or a combination of both drugs. Cell extracts were analyzed as in (A). (C) N87 cells grown in the presence of NDF were treated with the indicated concentrations of CI-1033, GA, Herceptin or specific combinations. Cell proliferation was examined using the MTT assay following 4 days of incubation. The results (averages ± SD of six determinations) are presented as the extent of cell growth relative to cultures maintained in the presence of NDF alone. (D) A431 cells were treated for 16 h with the indicated kinase inhibitors (at 20 µM each), GA (1 µM) or a mixture of GA and CI-1033, as indicated. Cell extracts were analyzed with the indicated antibodies. (E) A431 cells were treated with CI-1033 (10 µM) and GA (1 µM) or a combination of the drugs. Cell proliferation was assayed using the MTT assay over 4 days of cell treatment. The results (averages and SD of six determinations) are presented as the extent of cell growth relative to the cells at plating.
The effects of various TKIs, as well as GA, were also addressed in the ErbB-1-overexpressing human epidermoid tumor cell line A431. Prolonged treatment (16 h) with GA or high concentrations of CI-1033 (20 µM) resulted in enhanced ubiquitylation and degradation of ErbB-1, but PD201853 and AG1478, two ErbB-1-specific and reversible TKIs, caused smaller effects (Figure 7D). A combination of CI-1033 and GA only slightly enhanced receptor degradation, especially when relatively low drug concentrations were used (Figure 7D; data not shown). However, the combination of CI-1033 and GA exhibited reproducible additive effects on inhibition of growth of A431 cells, especially after relatively long intervals of incubation (Figure 7E). Taken together, these results support a model attributing to TKIs the ability to target receptor tyrosine kinases to a chaperone-associated destruction pathway, and they underscore potential therapeutic applications of TKIs in combination with other drugs.
Discussion
Comparison of the sensitivities of the four ErbB proteins to a chaperone antagonist (Figure 1) and thermal stress (Figure 5D) suggested that association with the Hsp90 chaperone is especially important for maintaining ErbB-2 in a signaling-competent form. This observation is in line with the differential heat stability (Liu and Carpenter, 1993) and the reported GA sensitivity (Xu et al., 2001a) of ErbB-1 and ErbB-2. In analogy to ErbB-2/HER2, the most transforming member of the family, oncogenic mutants of Src and Abl tyrosine kinases, which exhibit enhanced kinase activity, display higher GA sensitivity than their non-oncogenic and catalytically less active forms (An et al., 2000). A key finding made in the course of the present study is the ability of a mutation within the kinase domain of ErbB-1 to confer increased basal and GA-induced ubiquitylation and degradation of the receptor (Figure 1). Because more extensive structural alterations affecting non-catalytic portions of ErbB-1 were significantly less effective (Figure 1C), we inferred that the chaperone-mediated degradation machinery is particularly sensitive to the conformation of the ATP-binding site. We therefore examined the prediction that TKIs can mimic the effect of the kinase domain mutation, and thereby promote selective degradation of their target kinases. Screening representative TKIs identified an irreversible antagonist (CI-1033) as a potent inducer of poly-ubiquitylation and degradation of ErbB-2 (Figure 2A), supporting this prediction. It is important to note the lower activity of a reversible analog of the active compound. Nevertheless, upon relatively long treatments with high concentrations of reversible TKIs, we observed degradation of ErbB-2 and ErbB-1 (Figures 2A and 7D; data not shown), attributing an important role for drug-induced distortion of kinase conformation as a trigger for degradation. The high degree of specificity of irreversible TKIs to ErbB proteins has been shown previously (Fry et al., 1998; Smaill et al., 2001) and, as we have demonstrated, mutation of Cys805 in the kinase domain of ErbB-2 (Figure 4E) abolishes the activity of the inhibitor towards the receptor. Hence, the destructive activity of CI-1033 towards ErbB proteins is expected to be extremely specific, unlike the broad action of chaperone antagonists.
Apparently, two major inducible pathways control degradation of ErbB proteins. Ligand-induced rapid endocytosis of ErbB-1 (Levkowitz et al., 1999), as well as antibody- and oncogenic mutation-induced degradation of ErbB-2 (Klapper et al., 2000; Levkowitz et al., 2000), are mediated, at least in part, by the c-Cbl ubiquitin ligase. On the other hand, drug- or stress-induced degradation of ErbB-2 involves shuffling of chaperone complexes associated with the receptor (Figure 6C; Xu et al., 2001a), and subsequent endocytosis (Figure 2D) (Chavany et al., 1996), ubiquitylation and degradation, apparently mediated by proteasomal proteinases (Figure 2E and F). The Cbl-mediated pathway requires kinase activity and tyrosine phosphorylation at the C-terminal tails of ErbBs (Levkowitz et al., 1999; Klapper et al., 2000), whereas the chaperone-mediated route is recruited to the kinase domain, especially upon structural perturbation (Figure 4). In addition, only the membranal fraction of receptors is targeted by the ligand-dependent mechanism, whereas the chaperone-mediated pathway acts on both mature and immature receptors (Figure 3). Thus, the two degradative pathways appear functionally and structurally distinct, and they may fulfill complementary physiological roles.
Several lines of evidence indicate that CI-1033 directs ErbB-2 to a degradative fate mediated by chaperone complexes. First, the biochemical consequences of antagonizing Hsp90, a chaperone that controls ErbB-2 stability (Xu et al., 2001a), are similar to the action of TKIs on ErbB-2 degradation, and both treatments promote poly-ubiquitylation (Figure 3). Secondly, the physiological correlate of ErbB-2 exposure to an irreversible TKI, or to a chaperone antagonist, appears to be thermal stress (Figure 5). In addition, unlike the c-Cbl-mediated pathway, which favors ErbB-1, the CI-1033- and GA-induced pathways display a preference for ErbB-2. Lastly, ubiquitylation of ErbB-2 following treatment with either CI-1033 or GA appears to involve disruption of a complex containing Hsp90, recruitment of Hsp70 (Figure 6), and possibly also engagement of the co-chaperones Bag1, p50cdc37 and CHIP, whose U-box may mediate poly-ubiquitylation of ErbB-2 (our unpublished results).
Interestingly, CI-1033, GA and heat shock accelerate degradation of both the mature and the nascent forms of ErbB-2 (Figures 3 and 5), but degradation of the immature ER-localized form appears slower, and less extensive. Previous reports implicated a luminal, ER-resident chaperone, namely Grp94, in stabilizing the nascent form of ErbB-2 (Chavany et al., 1996; Mimnaugh et al., 1996), but the crucial role of the kinase domain in chaperone recognition favors interaction with Hsp90 already in the ER (Xu et al., 2001a). Along with their similarities, the effects of GA and CI-1033 on ErbB proteins differ in some aspects, including dependence on the integrity of the kinase domain (Figures 1A and 4). These observations led us to propose that CI-1033 identifies ErbB-2 to the chaperone-mediated destructive system through binding to and perturbing the ATP-binding pocket of the oncoprotein. In contrast, GA binds to and inactivates the ATP-binding pocket of Hsp90, thereby presenting ErbB-2 to the same destructive machinery (Figure 6C). Hence, independent of the priming agent, the two pathways converge to enhance poly-ubiquitylation and degradation of the receptor. This model can explain why a combination of CI-1033 and GA additively augments ErbB-2 degradation, and how the drugs consequently collaborate in arresting cell growth (Figure 7). Moreover, this interpretation suggests that TKIs, which act as degradation-inducing factors, combine the effectiveness of GA analogs with the high specificity of kinase inhibitors (Fry, 2000). Conceivably, the superior in vivo activity of irreversible TKIs (Vincent et al., 2000) is due to their combined action as kinase inhibitors and degradation-inducing factors. Additional benefits of the use of irreversible TKIs lie in prolonged pharmacological effects and lower toxicity due to covalent target binding and shorter periods of treatment. This, in turn, may open a time window for treatment with other agents (e.g. chemotherapy and radiotherapy), which take advantage when surface ErbB-2 is downregulated (Pegram et al., 1999; Pietras et al., 1999).
The therapeutic potential of understanding the mode of action of degradation-inducing TKIs is exemplified by the additive effect of CI-1033 and GA on inhibition of tumor cell growth (Figure 7). Moreover, lessons learned with ErbB-2 may be relevant for targeting other chaperone-controlled oncoproteins to destruction. Future studies will address the suitability of additional oncoproteins, but sensitivity to GA already discloses the candidacy of the insulin- and IGF-1 receptors, as well as Raf1 and Bcr-Abl (Schulte et al., 1995; Sepp Lorenzino et al., 1995; An et al., 2000). Since Hsp90 chaperones several other therapeutically attractive molecules, such as mutant p53 and steroid hormone receptors (reviewed in Buchner, 1999), it is conceivable that specific destruction of such oncoproteins may be achieved by utilizing target-specific drugs tailored to perturb recognition of these specific substrates by Hsp90. This novel approach may open the way for therapy of cancer and other diseases whose pathology depends on one or more chaperone-regulated proteins.
Materials and methods
Reagents and plasmids
CI-1033 and PD21853 were synthesized as described previously (Fry et al., 1998). GA was obtained from the Drug Synthesis and Chemistry Branch of the National Cancer Institute (Rockville, MD). Genistein, AG-1478, AG-1296 and lactacystin were from Calbiochem (La Jolla, CA). Herceptin was provided by Genentech Inc. (South San Francisco, CA). Antibodies were from Santa-Cruz Biotechnology, except for anti-Hsp90 and anti-Hsp70 (Stressgen), anti-ubiquitin (Babco and Sigma), anti-HA (Roche) and anti-Shc (Transduction Laboratories). Horseradish peroxidase (HRP)-conjugated streptavidin, HRP-conjugated IgGs and donkey anti-mouse Cy3-conjugated IgG were from Jackson Laboratories. EGF and Neu differentiation factor (NDF) were from Sigma (St Louis, MO) and Amgen (Thousand Oaks, CA), respectively. pcDNA3-based expression vectors encoding ErbB-1, ErbB-2, ErbB-3, ErbB-4 and a kinase-defective mutant of ErbB-1 (K721A) have been described previously (Levkowitz et al., 1999). An expression vector encoding HA-tagged ubiquitin (HA-Ub) was a gift from Dirk Bohmann (EBI, Heidelberg, Germany). An expression vector encoding HA-Ub in which all the lysine residues were replaced by arginine (Ub-KO) was a gift from Dr C.M.Pickart (Johns Hopkins University). The pCMV vector encoding a kinase-defective mutant of ErbB-2 (K753A) was provided by Wei-Zen Wei (Karmanos Cancer Institute, Detroit, MI). Mutation of Cys805 to alanine was carried out on pcDNA3 ErbB-2 using Quikchange mutagenesis (Stratagene).
Cell culture and transfection
T47D-5R cells (Beerli et al., 1994) were obtained from N.Hynes (FMI, Basel, Switzerland). Derivatives of 32D cells were established as described previously (Pinkas-Kramarski et al., 1996). Transient transfection of CHO, COS and HEK-293T cells was carried out using Lipofectamine (Life Technologies, Bethesda, MD), Fugene (Roche) or the calcium phosphate method, respectively.
Cell proliferation assays
N87 cells (5 × 104 cells/ml) were plated in 96-well plates in full medium in the presence of NDF. Proliferation was assayed by adding 3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyl tetrazolium bromide (MTT; final concentration 0.05 mg/ml) and incubating for 2 h. Signals were quantified by reading optical density at 540–630 nm after lysis of cells in acidic isopropanol.
Lysate preparation, immunoprecipitation and western blot analysis
Stock solutions of TKIs were prepared in dimethylsulfoxide (DMSO) or in water. Unless indicated otherwise, cells were scraped in buffer A (50 mM Na–HEPES pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1.5 mM MgCl2) containing fresh Na3VO4 (1 mM) and a mixture of protease inhibitors. Where specified, TMNS buffer (50 mM Tris–HCl, 150 mM NaCl, 20 mM NaMoO4, 0.09% NP-40) was used. Cell lysis, immunoprecipitation and western blotting were carried out essentially as described previously (Levkowitz et al., 1999). For biotinylation experiments, cells were incubated on ice for 40 min with Biotin-X-NHS (0.5 mg/ml in PBS; Calbiochem). Biotin was quenched by washes with a cold solution of 15 mM glycine in PBS.
Metabolic labeling of cultured cells
Cells were washed and incubated for 2 h in medium free of cysteine and methionine, followed by addition of 0.1 mCi/ml 35S-labeled cysteine and methionine (Amersham) for an overnight incubation (pulse). Cells were then washed thoroughly, incubated in media containing cysteine and methionine, and given the indicated treatment (chase). This was followed by cell lysis, immunoprecipitation, electrophoresis and autoradiography.
Immunofluorescence
N87 cells growing on glass slides were fixed in 3% paraformaldehyde for 15 min at room temperature. Cells were then permeabilized for 10 min with PBS containing Triton X-100 (0.2%) and albumin (1%). Slides were incubated with a mAb to ErbB-2 (L26; 33 ng/ml) for 1 h at room temperature, followed by incubation for 40 min with a CY3-conjugated donkey anti-mouse antibody.
Receptor downregulation assay
Cells grown in 24-well plates were incubated at 37°C with the indicated agents for various time intervals in binding buffer (0.1% albumin in DME medium containing 20 mM HEPES pH 7.5). Cells were then transferred to ice, and surface-bound antibody molecules were removed by use of low pH wash (Levkowitz et al., 1999). The level of receptor residing on the cell surface was then determined by incubating the cells at 4°C for 90 min with a radiolabeled mAb L26.
Acknowledgments
Acknowledgements
We thank Dirk Bohmann for HA-Ub plasmid, Nancy Hynes for T47D-5R cells, Cecile Pickart for KO-Ub and Wei-Zen Wei for a mutant ErbB-2 plasmid. This work was supported by the National Cancer Institute (grants CA72981 to Y.Y. and HL03658 to C.P.), a grant from Genentech Inc. and a generous contribution made by Dr Marvin Klein.
References
- An W.G., Schulte,T.W. and Neckers,L.M. (2000) The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ., 11, 355–360. [PubMed] [Google Scholar]
- Beerli R.R., Wels,W. and Hynes,N.E. (1994) Intracellular expression of single chain antibodies reverts ErbB-2 transformation. J. Biol. Chem., 269, 23931–23936. [PubMed] [Google Scholar]
- Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci., 24, 136–141. [DOI] [PubMed] [Google Scholar]
- Chavany C. et al. (1996) p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem., 271, 4974–4977. [DOI] [PubMed] [Google Scholar]
- Fry D.W. (2000) Site-directed irreversible inhibitors of the erbB family of receptor tyrosine kinases as novel chemotherapeutic agents for cancer. Anticancer Drug Des., 15, 3–16. [PubMed] [Google Scholar]
- Fry D.W. et al. (1998) Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl Acad. Sci. USA, 95, 12022–12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Chen,J., App,H., McMahon,G., Hirth,P., Chen,I. and Levitzki,A. (1996) Tyrphostins IV—highly potent inhibitors of EGF receptor kinase. Structure–activity relationship study of 4-anilidoquinazolines. Bioorg. Med. Chem., 4, 1203–1207. [DOI] [PubMed] [Google Scholar]
- Kasprzyk P.G., Song,S.U., Di Fiore,P.P. and King,C.R. (1992) Therapy of an animal model of human gastric cancer using a combination of anti-ErbB-2 monoclonal antibodies. Cancer Res., 52, 2771–2776. [PubMed] [Google Scholar]
- Klapper L.N., Vaisman,N., Hurwitz,E., Pinkas Kramarski,R., Yarden,Y. and Sela,M. (1997) A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene, 14, 2099–2109. [DOI] [PubMed] [Google Scholar]
- Klapper L.N., Waterman,H., Sela,M. and Yarden,Y. (2000) Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res., 60, 3384–3388. [PubMed] [Google Scholar]
- Levitzki A. (1999) Protein tyrosine kinase inhibitors as novel therapeutic agents. Pharmacol. Ther., 82, 231–239. [DOI] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Oved,S., Klapper,L.N., Harari,D., Lavi,S., Sela,M. and Yarden,Y. (2000) c-Cbl is a suppressor of the neu oncogene. J. Biol. Chem., 275, 35532–35539. [DOI] [PubMed] [Google Scholar]
- Liu S.M. and Carpenter,G. (1993) Differential heat stress stability of epidermal growth factor receptor and ErbB-2 receptor tyrosine kinase activities. J. Cell Physiol., 157, 237–242. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E.G., Chavany,C. and Neckers,L. (1996) Polyubiquitination and proteasomal degradation of the p185c-ErbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem., 271, 22796–22801. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Mizuno,S. and Uehara,Y. (1994) Accelerated degradation of 160 kDa epidermal growth factor (EGF) receptor precursor by the tyrosine kinase inhibitor herbimycin A in the endoplasmic reticulum of A431 human epidermoid carcinoma cells. Biochem. J., 301, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L., Mimnaugh,E. and Schulte,T. (1999) The Function of Heat Shock Proteins in Stress Tolerance: Degradation and Reactivation of Damaged Proteins. Springer-Verlag, Heidelberg, Germany.
- Pegram M. and Slamon,D. (2000) Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. Semin. Oncol., 27, 13–19. [PubMed] [Google Scholar]
- Pegram M. et al. (1999) Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene, 18, 2241–2251. [DOI] [PubMed] [Google Scholar]
- Pietras R.J., Poen,J.C., Gallardo,D., Wongvipat,P.N., Lee,H.J. and Slamon,D.J. (1999) Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res., 59, 1347–1355. [PubMed] [Google Scholar]
- Pinkas-Kramarski R. et al. (1996) Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J., 15, 2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Schulte T.W., Blagosklonny,M.V., Ingui,C. and Neckers,L. (1995) Disruption of the Raf-1–Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1–Ras association. J. Biol. Chem., 270, 24585–24588. [DOI] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Ma,Z., Lebwohl,D.E., Vinitsky,A. and Rosen,N. (1995) Herbimycin A induces the 20 S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J. Biol. Chem., 270, 16580–16587. [DOI] [PubMed] [Google Scholar]
- Slamon D.J. et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 244, 707–712. [DOI] [PubMed] [Google Scholar]
- Smaill J.B. et al. (2001) Tyrosine kinase inhibitors. 18. 6-substituted 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as soluble, irreversible inhibitors of the epidermal growth factor receptor. J. Med. Chem., 44, 429–440. [DOI] [PubMed] [Google Scholar]
- Thrower J.S., Hoffman,L., Rechsteiner,M. and Pickart,C.M. (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J., 19, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhomirov O. and Carpenter,G. (2000) Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J. Biol. Chem., 275, 26625–26631. [DOI] [PubMed] [Google Scholar]
- Vincent P.W. et al. (2000) Anticancer efficacy of the irreversible EGFr tyrosine kinase inhibitor PD 0169414 against human tumor xenografts. Cancer Chemother. Pharmacol., 45, 231–238. [DOI] [PubMed] [Google Scholar]
- Xu W., Mimnaugh,E., Rosser,M.F., Nicchitta,C., Marcu,M., Yarden,Y. and Neckers,L. (2001a) Sensitivity of mature ErbB2 to geldanamycin is conferred by its kinase domain and is mediated by chaperone protein Hsp90. J. Biol. Chem., 276, 3702–3708. [DOI] [PubMed] [Google Scholar]
- Xu W., Mimnaugh,E.G., Kim,J.-S., Trepel,J.B. and Neckers,L.M. (2001b) Hsp90, not Grp94, regulates the intracellular trafficking and stabilty of nascent ErbB2. Cell Stress Chaperones, 7, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y. and Sliwkowski,M.X. (2001) Untangling the ErbB signalling network. Nature Rev. Mol. Cell. Biol., 2, 127–137. [DOI] [PubMed] [Google Scholar]
- Zheng F.F., Kuduk,S.D., Chiosis,G., Munster,P.N., Sepp-Lorenzino,L., Danishefsky,S.J. and Rosen,N. (2000) Identification of a gelda namycin dimer that induces the selective degradation of HER-family tyrosine kinases. Cancer Res., 60, 2090–2094. [PubMed] [Google Scholar]