Abstract
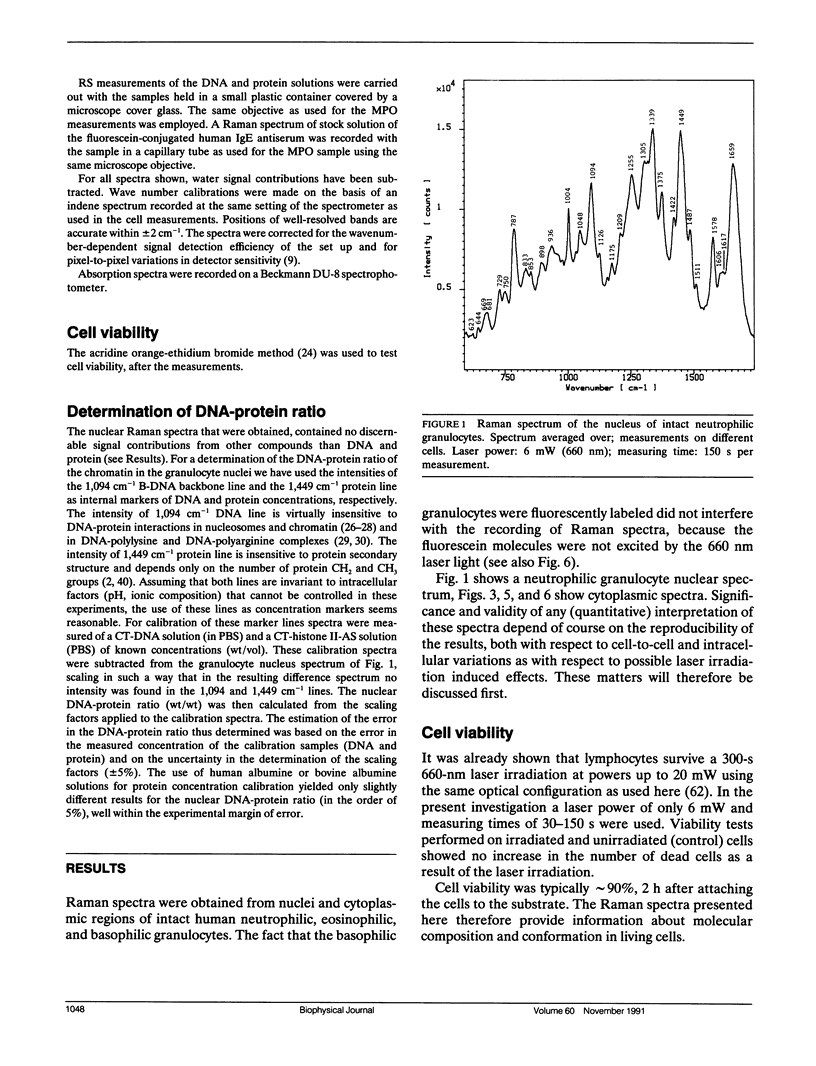
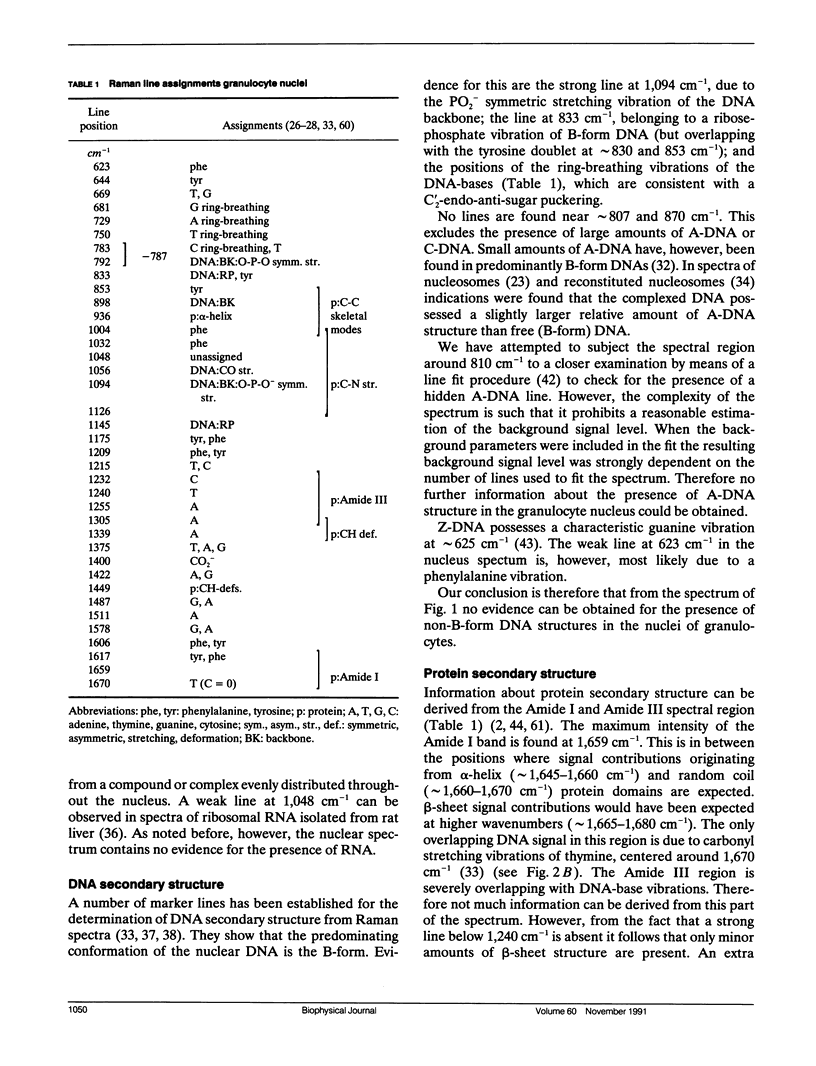
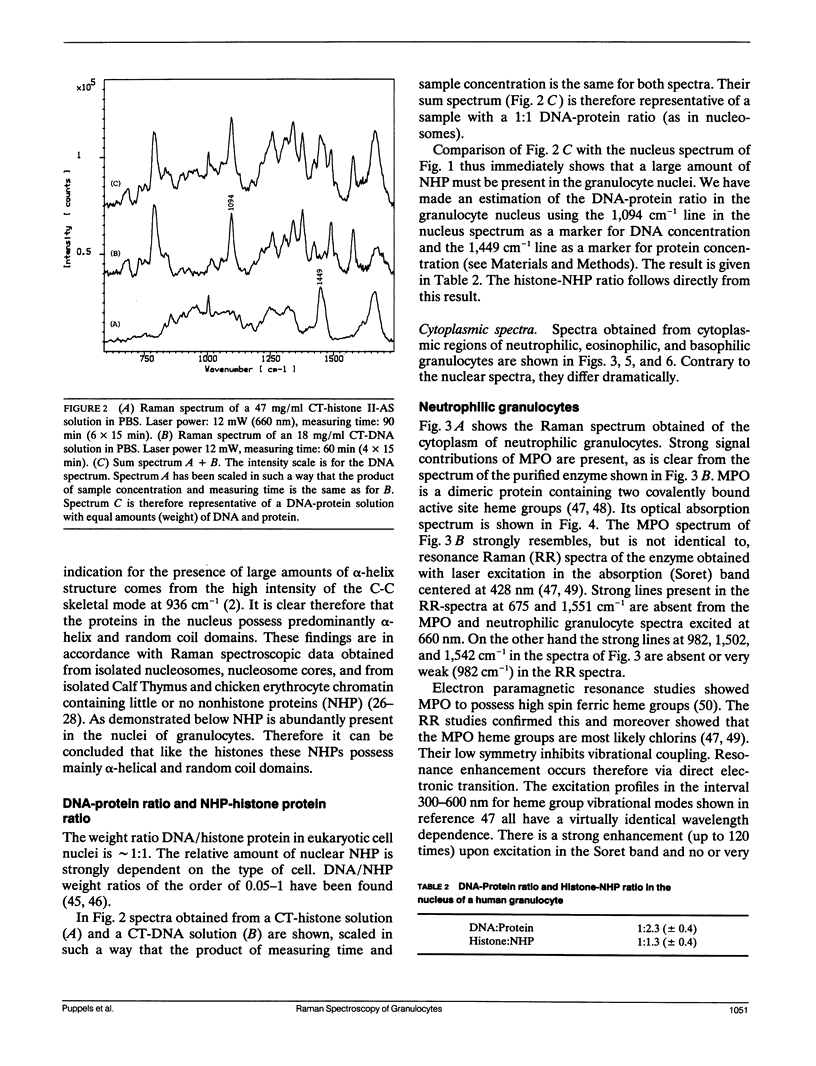
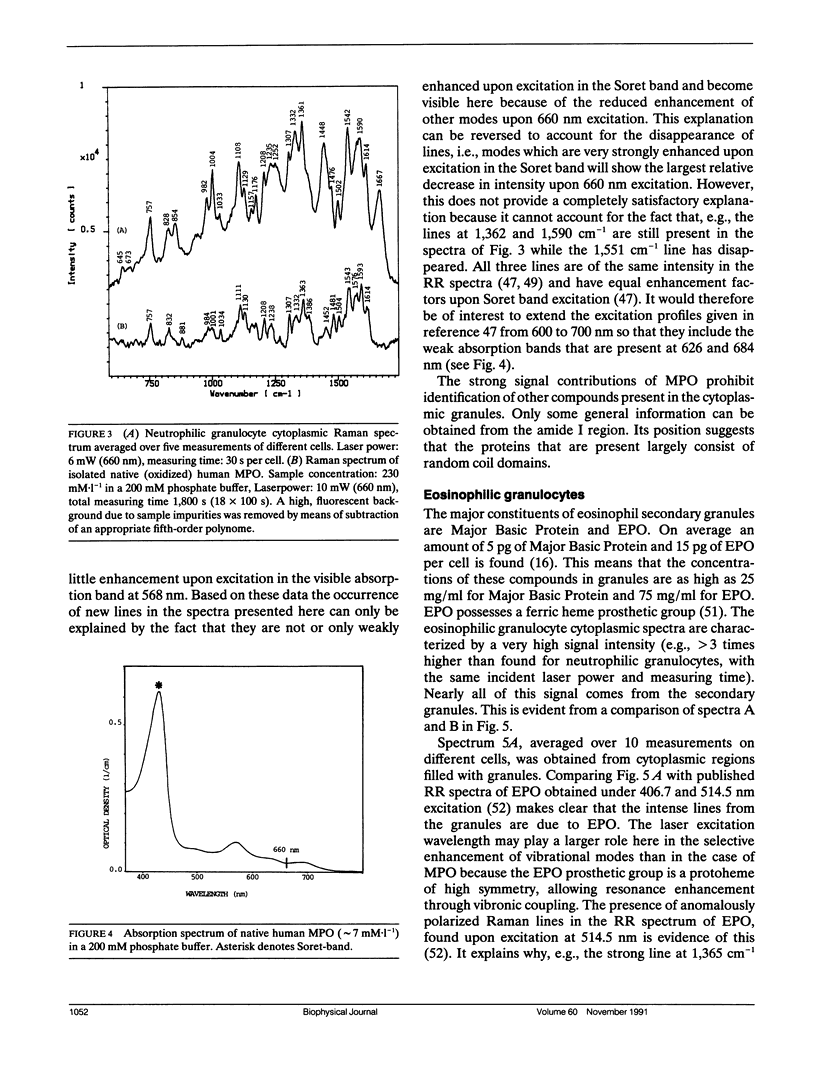
A sensitive confocal Raman microspectrometer was employed to record spectra of nuclei and cytoplasmic regions of single living human granulocytes. Conditions were used that ensured cell viability and reproducibility of the spectra. Identical spectra were obtained from the nuclei of neutrophilic, eosinophilic, and basophilic granulocytes, which yield information about DNA and protein secondary structure and DNA-protein ratio. The cytoplasmic Raman spectra of the three cell types are very different. This was found to be mainly due to the abundant presence of peroxidases in the cytoplasmic granules of neutrophilic granulocytes (myeloperoxidase) and eosinophilic granulocytes (eosinophil peroxidase). Strong signal contributions of the active site heme group(s) of these enzymes were found. This paper illustrates the potentials and limitations for Raman spectroscopic analysis of cellular constituents and processes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. J., Kephart G. M., Habermann T. M., Greipp P. R., Gleich G. J. Localization of eosinophil granule major basic protein in human basophils. J Exp Med. 1983 Sep 1;158(3):946–961. doi: 10.1084/jem.158.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Ingle R. T., Oertling W. A., Davis J. C., Averill B. A., Hulse C. L., Stufkens D. J., Bolscher B. G., Wever R. Raman characterization of human leukocyte myeloperoxidase and bovine spleen green haemoprotein. Insight into chromophore structure and evidence that the chromophores of myeloperoxidase are equivalent. Biochim Biophys Acta. 1985 Mar 22;828(1):58–66. doi: 10.1016/0167-4838(85)90009-3. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Bansil R., Yannas I. V., Stanley H. E. Raman spectroscopy: a structural probe of glycosaminoglycans. Biochim Biophys Acta. 1978 Jul 17;541(4):535–542. doi: 10.1016/0304-4165(78)90163-0. [DOI] [PubMed] [Google Scholar]
- Barry B., Mathies R. Resonance Raman microscopy of rod and cone photoreceptors. J Cell Biol. 1982 Aug;94(2):479–482. doi: 10.1083/jcb.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms S., Brahmachari S. K., Angelier N., Brahms J. G. Conformation of DNA in chromatin reconstituted from poly [d(A-T)] and the core histones. Nucleic Acids Res. 1981 Oct 10;9(19):4879–4893. doi: 10.1093/nar/9.19.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield J. H., Maddox D. E., Gleich G. J. The eosinophil leukocyte: maturation and function. Clin Immunol Rev. 1983;2(2):187–306. [PubMed] [Google Scholar]
- Dvorak A. M., Ackerman S. J. Ultrastructural localization of the Charcot-Leyden crystal protein (lysophospholipase) to granules and intragranular crystals in mature human basophils. Lab Invest. 1989 Apr;60(4):557–567. [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- Goheen S. C., Gilman T. H., Kauffman J. W., Garvin J. E. The effect of Raman spectra of extraction of peripheral proteins from human erythrocyte membranes. Biochem Biophys Res Commun. 1977 Dec 7;79(3):805–814. doi: 10.1016/0006-291x(77)91183-4. [DOI] [PubMed] [Google Scholar]
- Goodwin D. C., Brahms J. Form of DNA and the nature of interactions with proteins in chromatin. Nucleic Acids Res. 1978 Mar;5(3):835–850. doi: 10.1093/nar/5.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Nishimura Y., Katahira M., Tsuboi M. The structure of nucleosome core particles as revealed by difference Raman spectroscopy. Nucleic Acids Res. 1986 Mar 25;14(6):2583–2596. doi: 10.1093/nar/14.6.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda-Saito M., Argade P. V., Rousseau D. L. Resonance Raman evidence of chloride binding to the heme iron in myeloperoxidase. FEBS Lett. 1985 May 6;184(1):52–55. doi: 10.1016/0014-5793(85)80651-7. [DOI] [PubMed] [Google Scholar]
- Kubasek W. L., Wang Y., Thomas G. A., Patapoff T. W., Schoenwaelder K. H., Van der Sande J. H., Peticolas W. L. Raman spectra of the model B-DNA oligomer d(CGCGAATTCGCG)2 and of the DNA in living salmon sperm show that both have very similar B-type conformations. Biochemistry. 1986 Nov 18;25(23):7440–7445. doi: 10.1021/bi00371a028. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Gorczyca L. E., Meiklejohn G. A laser Raman spectroscopic investigation of phospholipid and protein configurations in hemoglobin-free erythrocyte ghosts. Biochim Biophys Acta. 1975 Feb 28;382(1):51–57. doi: 10.1016/0005-2736(75)90371-5. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Tyminski D., Desmeules P. J. Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc. 1976 Oct 27;98(22):7075–7080. doi: 10.1021/ja00438a057. [DOI] [PubMed] [Google Scholar]
- Mansy S., Engstrom S. K., Peticolas W. L. Laser Raman identification of an interaction site on DNA for arginine containing histones in chromatin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1242–1247. doi: 10.1016/0006-291x(76)90330-2. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Prescott B., Steinmetz W., Thomas G. J., Jr Characterization of DNA structures by laser Raman spectroscopy. Biopolymers. 1984 Feb;23(2):235–256. doi: 10.1002/bip.360230206. [DOI] [PubMed] [Google Scholar]
- Puppels G. J., de Mul F. F., Otto C., Greve J., Robert-Nicoud M., Arndt-Jovin D. J., Jovin T. M. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature. 1990 Sep 20;347(6290):301–303. doi: 10.1038/347301a0. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Savoie R., Jutier J. J., Alex S., Nadeau P., Lewis P. N. Laser Raman spectra of calf thymus chromatin and its constituents. Biophys J. 1985 Apr;47(4):451–459. doi: 10.1016/S0006-3495(85)83937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbett S. S., Hurst J. K. Structural analysis of myeloperoxidase by resonance Raman spectroscopy. Biochemistry. 1984 Jun 19;23(13):3007–3013. doi: 10.1021/bi00308a025. [DOI] [PubMed] [Google Scholar]
- Sibbett S. S., Klebanoff S. J., Hurst J. K. Resonance Raman characterization of the heme prosthetic group in eosinophil peroxidase. FEBS Lett. 1985 Sep 23;189(2):271–275. doi: 10.1016/0014-5793(85)81038-3. [DOI] [PubMed] [Google Scholar]
- Stump R. F., Deanin G. G., Oliver J. M., Shelnutt J. A. Heme-linked ionizations of myeloperoxidase detected by Raman difference spectroscopy. A comparison with plant and yeast peroxidases. Biophys J. 1987 Apr;51(4):605–610. doi: 10.1016/S0006-3495(87)83385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstappen L. W., Mickaels R. A., Dost R., Loken M. R. Increased light scattering resolution facilitates multidimensional flow cytometric analysis. Cytometry. 1990;11(4):506–512. doi: 10.1002/cyto.990110409. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., de Grooth B. G., Nolten G. M., ten Napel C. H., van Berkel W., Greve J. Physical discrimination between human T-lymphocyte subpopulations by means of light scattering, revealing two populations of T8-positive cells. Cytometry. 1986 Mar;7(2):178–183. doi: 10.1002/cyto.990070209. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Hartman K. A. Raman studies of nucleic acids. 8. Estimation of RNA secondary structure from Raman scattering by phosphate-group vibrations. Biochim Biophys Acta. 1973 Jun 23;312(2):311–332. doi: 10.1016/0005-2787(73)90376-6. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Day L. A. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1 and Xf. Investigation by laser Raman spectroscopy. J Mol Biol. 1983 Apr 5;165(2):321–356. doi: 10.1016/s0022-2836(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Hamilton M. G. Raman spectra and conformational properties of ribosomes during various stages of disassembly. Biochemistry. 1980 Jul 22;19(15):3604–3613. doi: 10.1021/bi00556a029. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Olins D. E. Secondary structure of histones and DNA in chromatin. Science. 1977 Jul 22;197(4301):385–388. doi: 10.1126/science.560060. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Verma S. P. Raman and resonance-Raman scattering by erythrocyte ghosts. Biochim Biophys Acta. 1975 Apr 8;382(4):542–551. doi: 10.1016/0005-2736(75)90221-7. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Harrell J. T. Characteristics and variations of B-type DNA conformations in solution: a quantitative analysis of Raman band intensities of eight DNAs. Biochemistry. 1986 May 6;25(9):2664–2671. doi: 10.1021/bi00357a056. [DOI] [PubMed] [Google Scholar]
- Wever R., Hamers M. N., Weening R. S., Roos D. Characterization of the peroxidase in human eosinophils. Eur J Biochem. 1980 Jul;108(2):491–495. doi: 10.1111/j.1432-1033.1980.tb04746.x. [DOI] [PubMed] [Google Scholar]
- Wever R., Roos D., Weening R. S., Vulsma T., Van Gelder B. F. An EPR study of myeloperoxidase in human granulocytes. Biochim Biophys Acta. 1976 Feb 24;421(2):328–333. doi: 10.1016/0304-4165(76)90299-3. [DOI] [PubMed] [Google Scholar]


