Abstract
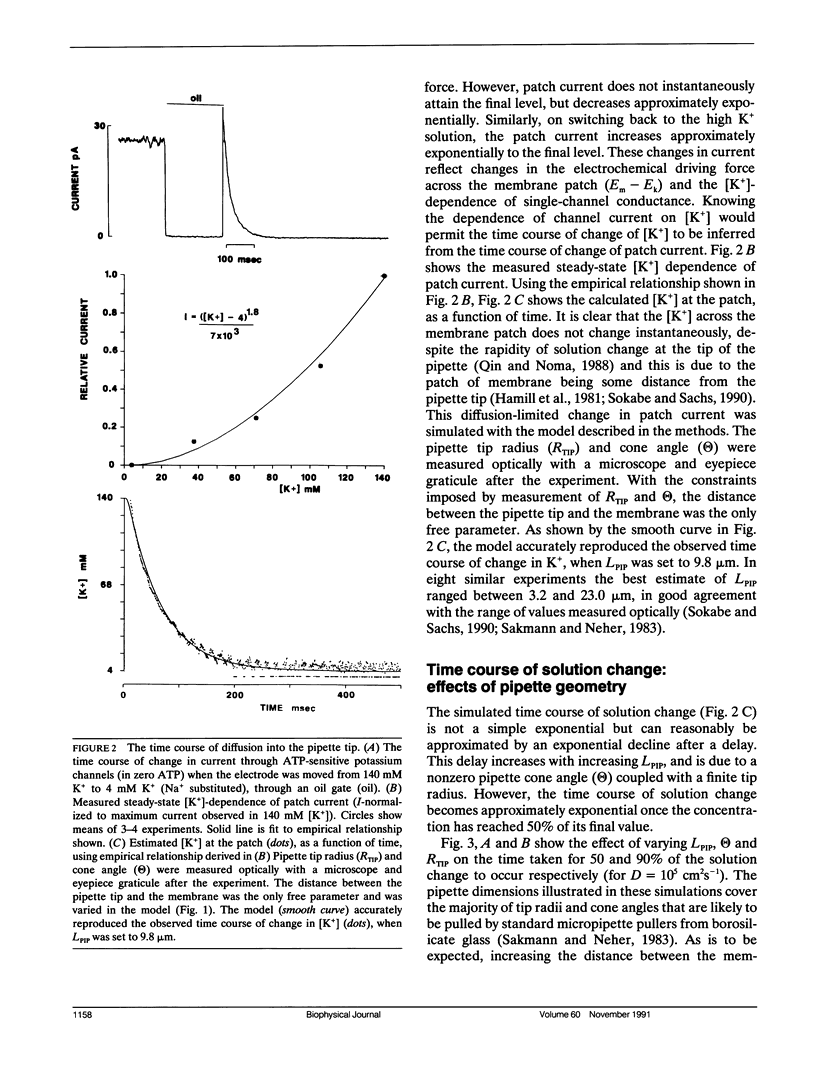
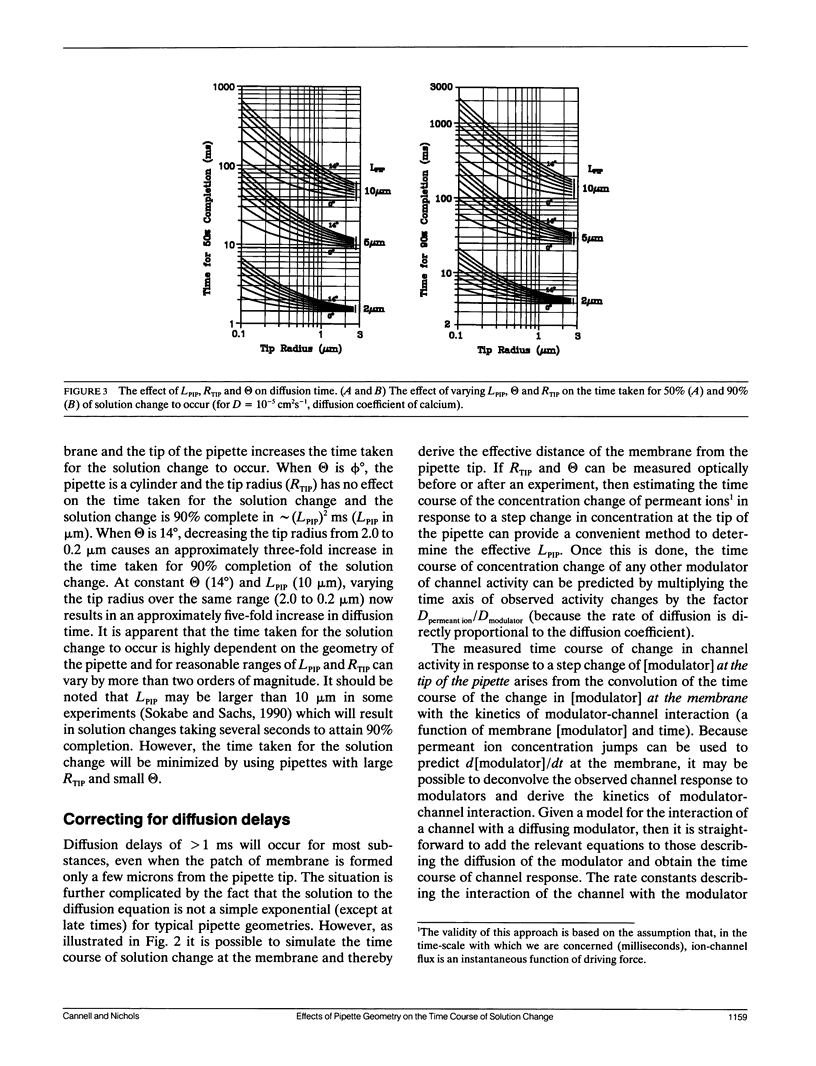
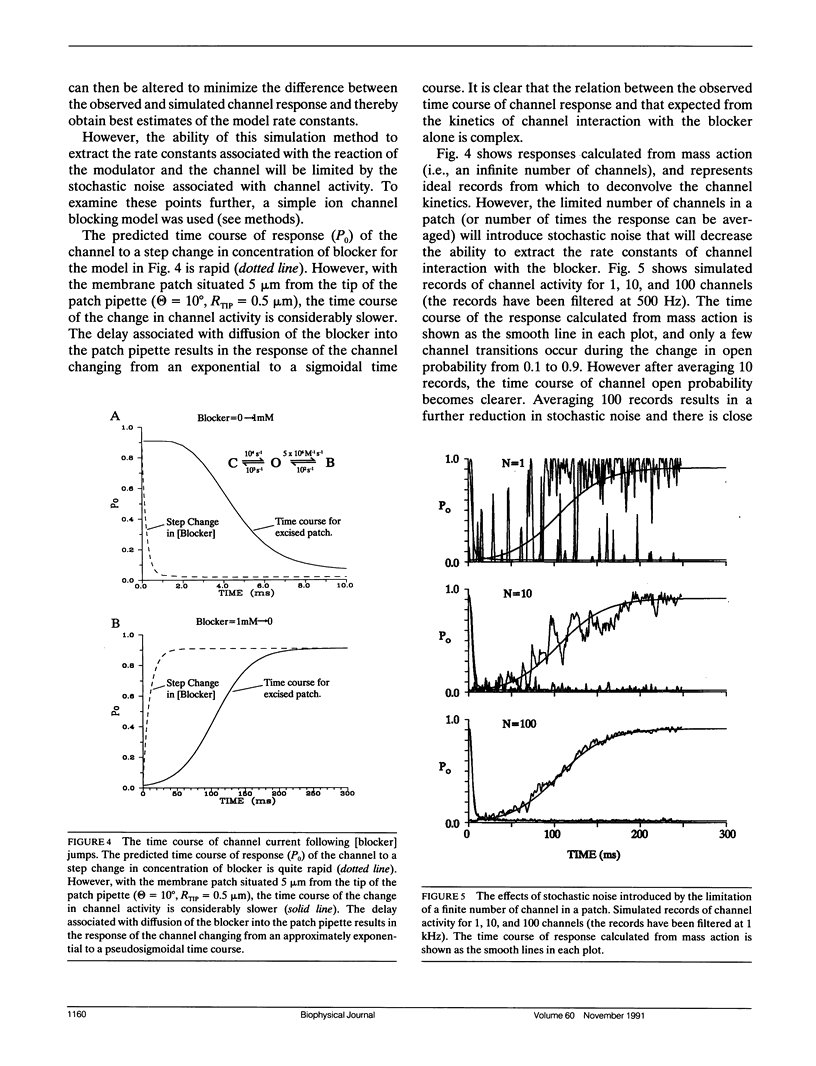
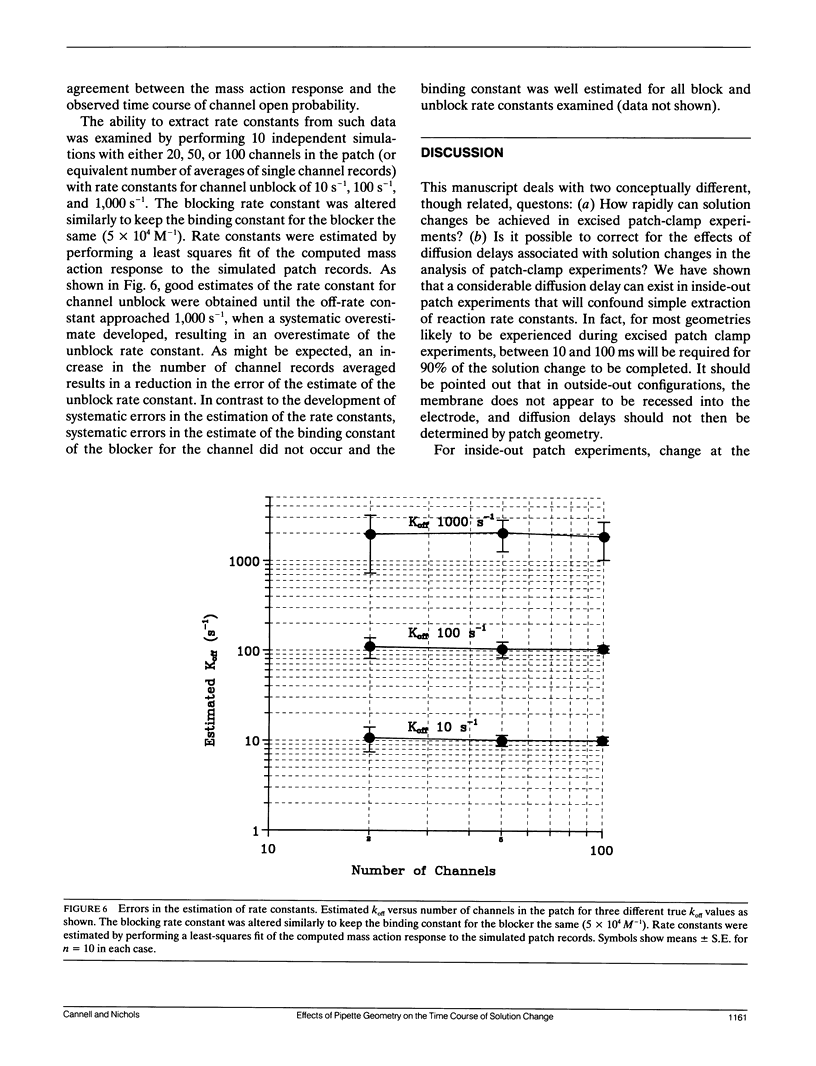
The time course of change in current through KATP channels in inside-out membrane patches, after step change of permeant ion (K+) concentration, was measured. A simple model of the patch as a membrane disc at the base of a cone with the apex removed, was able to describe the time course of channel activity after step change of [K+]. By measuring pipette geometry and using jumps of [permeant ion], it was then possible to estimate the time course of concentration at the membrane for jumps of any other ion or gating ligand. A simple channel block mechanism was used to simulate experiments with concentration jumps of a blocking ligand. The rate constants for ligand-channel interaction were extracted by least-squares fitting of computed mass action responses to those observed in simulated experiments. The simulations showed that even with diffusion delays of hundreds of milliseconds (as may occur in inside-out patch experiments), ligand association and dissociation rates of up to 1,000 s-1 could be accurately extracted by this approach. The approach should be generally applicable to the analysis of ligand concentration jump experiments on any ion channel whose activity is modulated by intracellular ligand.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Estimating kinetic parameters for single channels with simulation. A general method that resolves the missed event problem and accounts for noise. Biophys J. 1990 Dec;58(6):1411–1426. doi: 10.1016/S0006-3495(90)82487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D. Y., Noma A. A new oil-gate concentration jump technique applied to inside-out patch-clamp recording. Am J Physiol. 1988 Oct;255(4 Pt 2):H980–H984. doi: 10.1152/ajpheart.1988.255.4.H980. [DOI] [PubMed] [Google Scholar]
- Sokabe M., Sachs F. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. J Cell Biol. 1990 Aug;111(2):599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]



