Abstract
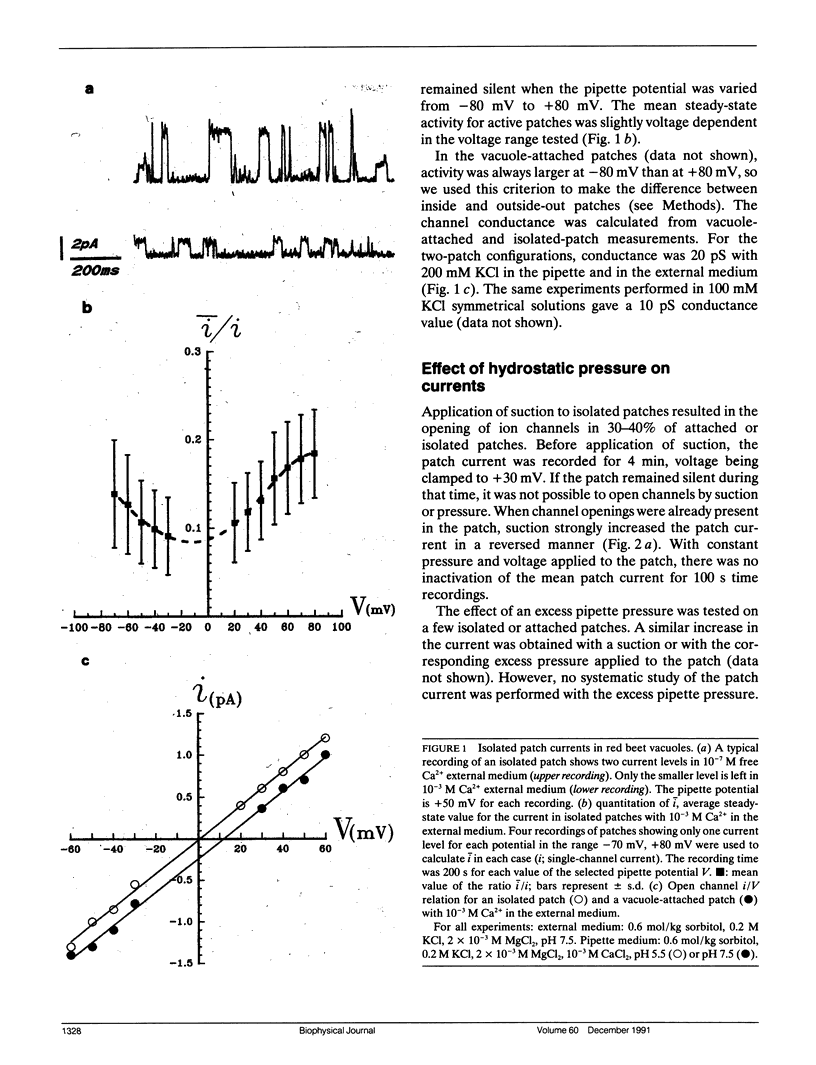
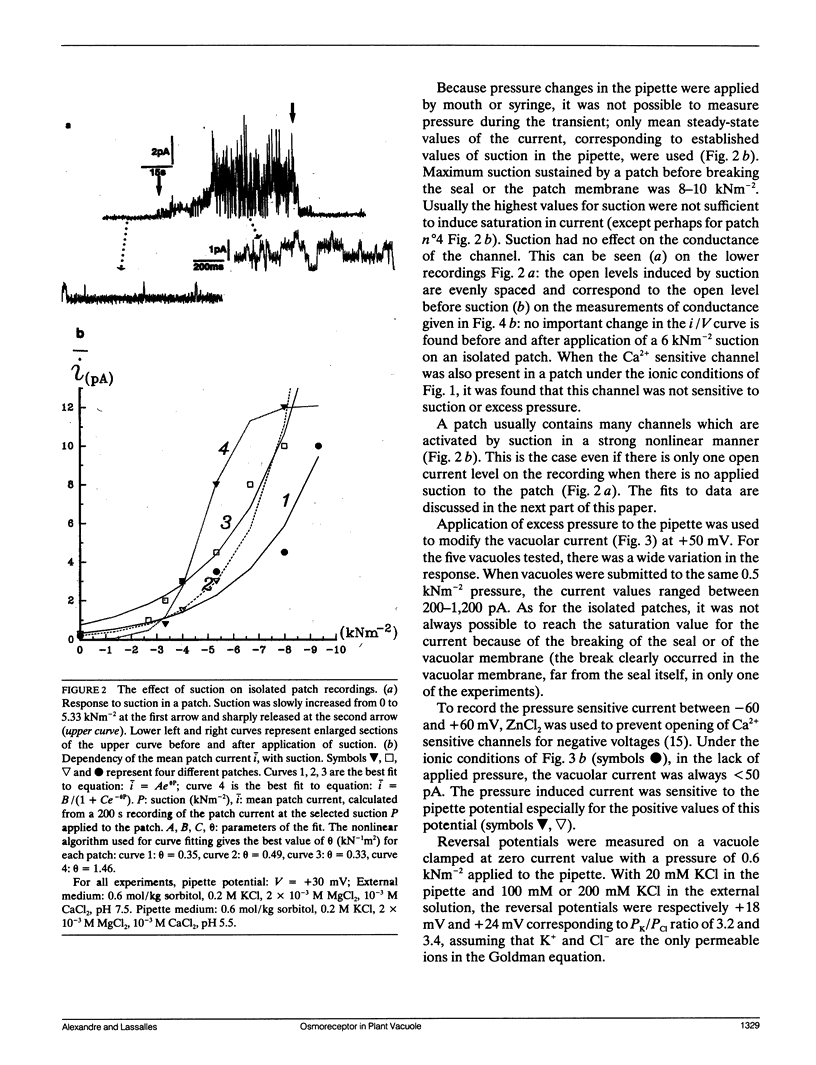
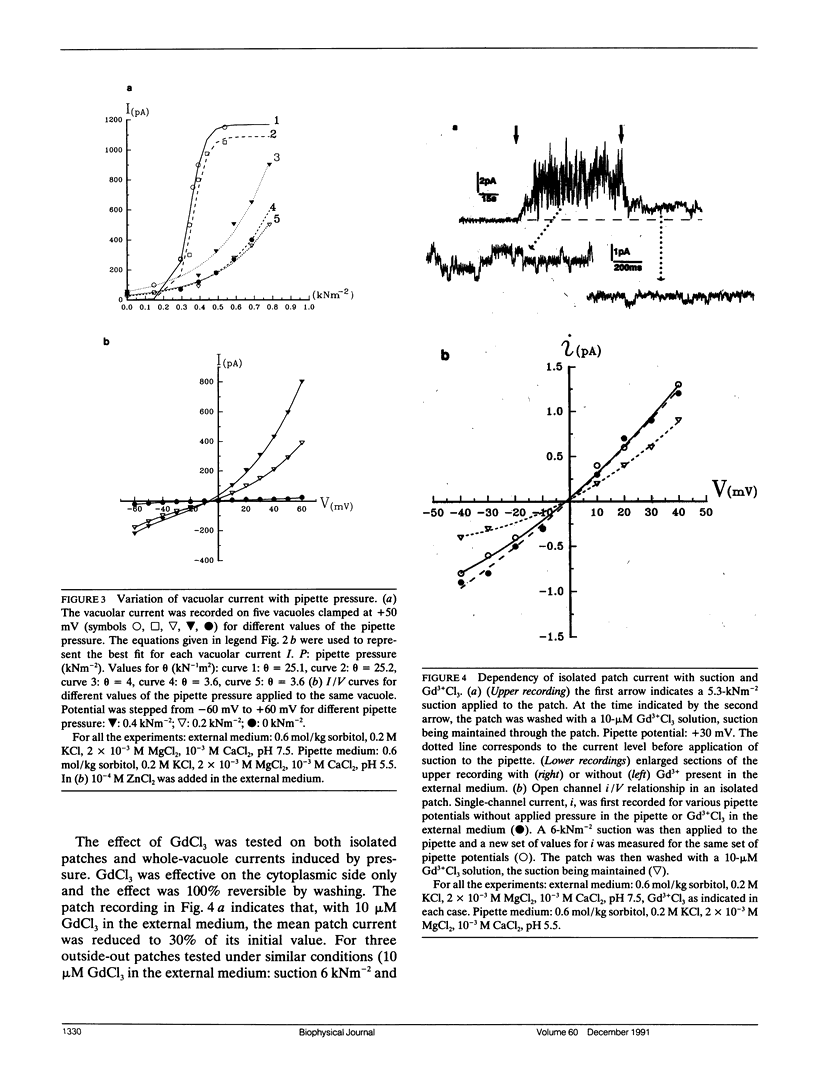
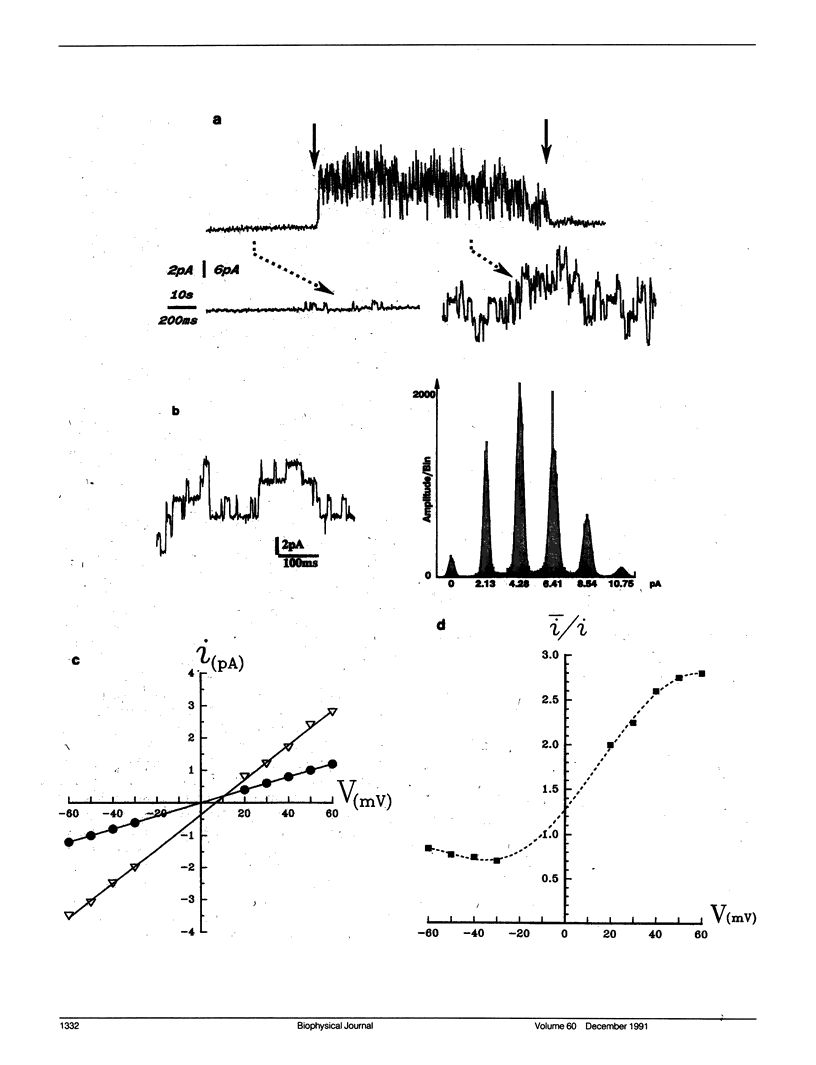
The vacuolar membrane of red beet vacuoles contains a channel which was not gated by voltage or Ca2+ ions. Its unit conductance was 20 pS in 200 mM symmetrical KCl solutions. It was stretch activated: the conductance remained constant but the probability of opening was increased by suction or pressure applied to a membrane patch. A 1.5-kNm-2 suction applied to isolated patches or a 0.08-kNm-2 pressure applied to a 45-μm diameter vacuole induced an e-fold change in the mean current. A 75% inhibition of the channel current was obtained with 10 μM Gd3+ on the cytoplasmic side. The channel was more permeable for K+ than for Cl- (PK/PCl ∼ 3). A possible clustering for this channel was suggested by the recordings of the patch current. The channel properties were not significantly affected by a change in sorbitol osmolality in the solutions under isoosmotic conditions, between 0.6 and 1 mol/kg sorbitol. However, the channel was very sensitive to an osmotic gradient. A 0.2-mol/kg sorbitol gradient induced a two-fold increase in unit conductance and a thirty-fold increase in the mean patch current of the channel. A current was measured, when the osmotic gradient was the only driving force applied to the vacuolar membrane. The hydrostatic and osmotic pressure (HOP) activated channel described in this paper could be gated in vivo condition by a change in osmolality, without the need of a change in the turgor pressure in the cell. The HOP channel represents a possible example of an osmoreceptor for plant cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke L. C., Edwards K. L., Pickard B. G., Misler S. A stretch-activated anion channel in tobacco protoplasts. FEBS Lett. 1988 Sep 12;237(1-2):141–144. doi: 10.1016/0014-5793(88)80188-1. [DOI] [PubMed] [Google Scholar]
- Franco A., Jr, Lansman J. B. Stretch-sensitive channels in developing muscle cells from a mouse cell line. J Physiol. 1990 Aug;427:361–380. doi: 10.1113/jphysiol.1990.sp018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Zhou X. L., Martinac B., Kung C. A mechanosensitive ion channel in the yeast plasma membrane. Science. 1988 Nov 4;242(4879):762–765. doi: 10.1126/science.2460920. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Roberts W. M., Hudspeth A. J. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem. 1988;17:99–124. doi: 10.1146/annurev.bb.17.060188.000531. [DOI] [PubMed] [Google Scholar]
- Ito T., Yamazaki M., Ohnishi S. Osmoelastic coupling in biological structures: a comprehensive thermodynamic analysis of the osmotic response of phospholipid vesicles and a reevaluation of the "dehydration force" theory. Biochemistry. 1989 Jun 27;28(13):5626–5630. doi: 10.1021/bi00439a043. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis F. J., Prins H. B. Patch clamp studies on root cell vacuoles of a salt-tolerant and a salt-sensitive plantago species : regulation of channel activity by salt stress. Plant Physiol. 1990 Jan;92(1):23–28. doi: 10.1104/pp.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990 Nov 15;348(6298):261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton R. L., Caldwell J. H. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 1990 Aug;416(6):758–762. doi: 10.1007/BF00370626. [DOI] [PubMed] [Google Scholar]
- Mittman S., Flaming D. G., Copenhagen D. R., Belgum J. H. Bubble pressure measurement of micropipet tip outer diameter. J Neurosci Methods. 1987 Dec;22(2):161–166. doi: 10.1016/0165-0270(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Interaction of ions and water in gramicidin A channels: streaming potentials across lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):327–340. doi: 10.1085/jgp.72.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Water permeability of gramicidin A-treated lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):341–350. doi: 10.1085/jgp.72.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Lecar H. Stochastic models for mechanical transduction. Biophys J. 1991 May;59(5):1143–1145. doi: 10.1016/S0006-3495(91)82329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16(2):141–169. [PubMed] [Google Scholar]
- Sokabe M., Sachs F., Jing Z. Q. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J. 1991 Mar;59(3):722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M., Sachs F. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. J Cell Biol. 1990 Aug;111(2):599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Ohnishi S., Ito T. Osmoelastic coupling in biological structures: decrease in membrane fluidity and osmophobic association of phospholipid vesicles in response to osmotic stress. Biochemistry. 1989 May 2;28(9):3710–3715. doi: 10.1021/bi00435a013. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Bezanilla F., Parsegian V. A. Solute inaccessible aqueous volume changes during opening of the potassium channel of the squid giant axon. Biophys J. 1990 May;57(5):1049–1064. doi: 10.1016/S0006-3495(90)82623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


