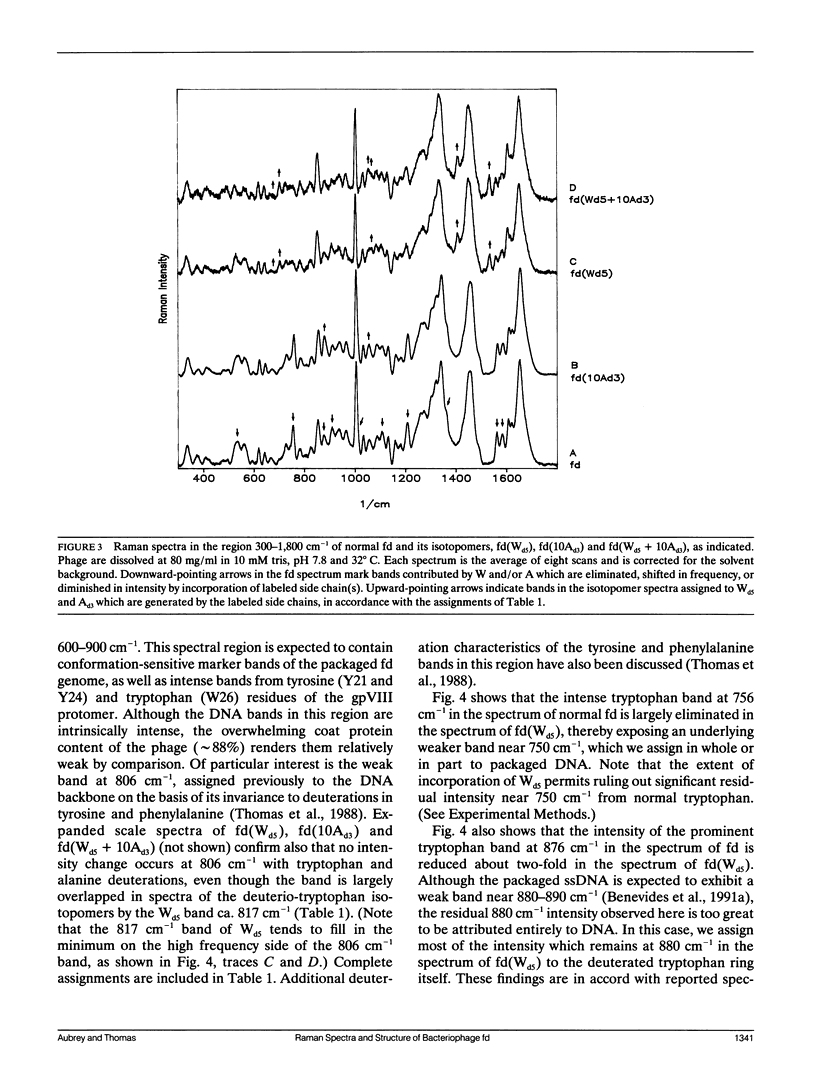
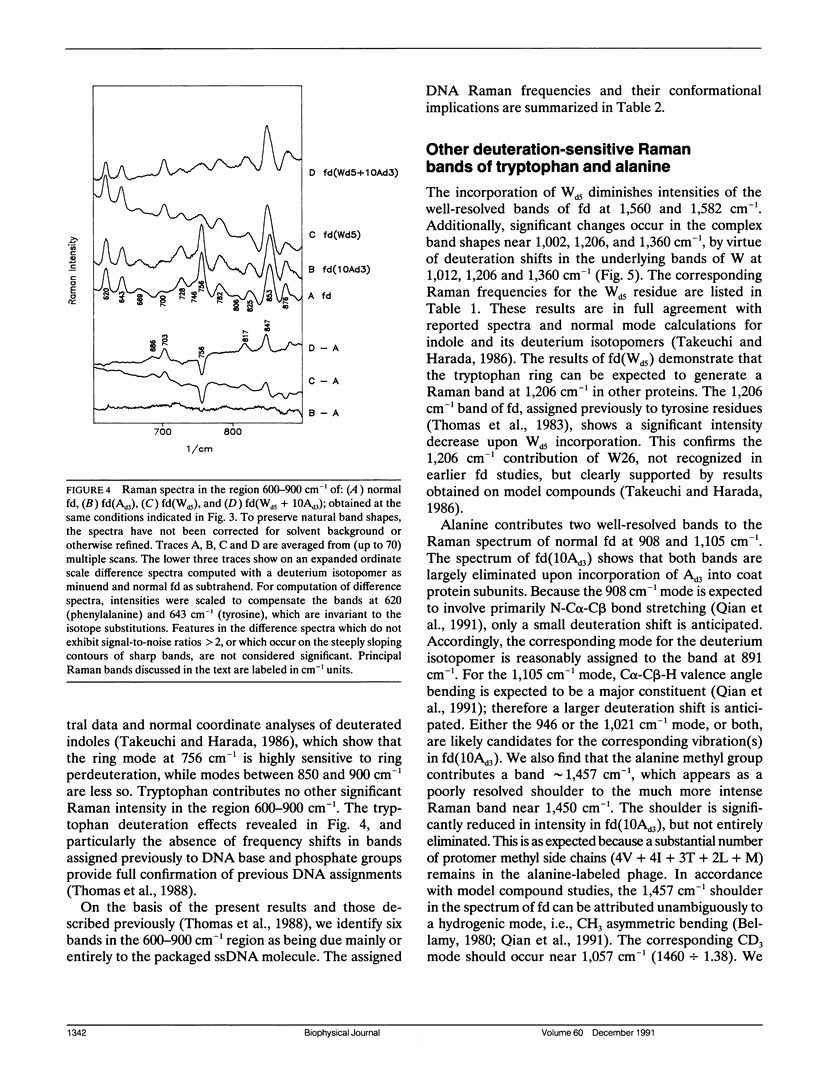
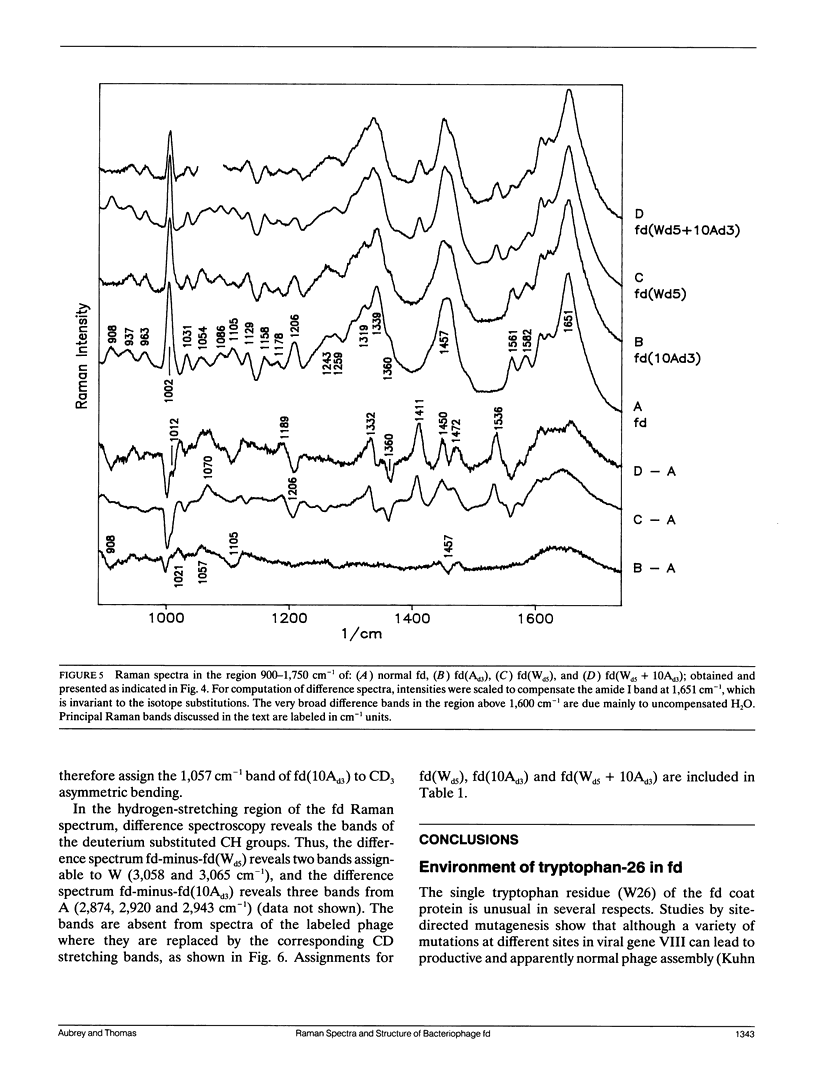
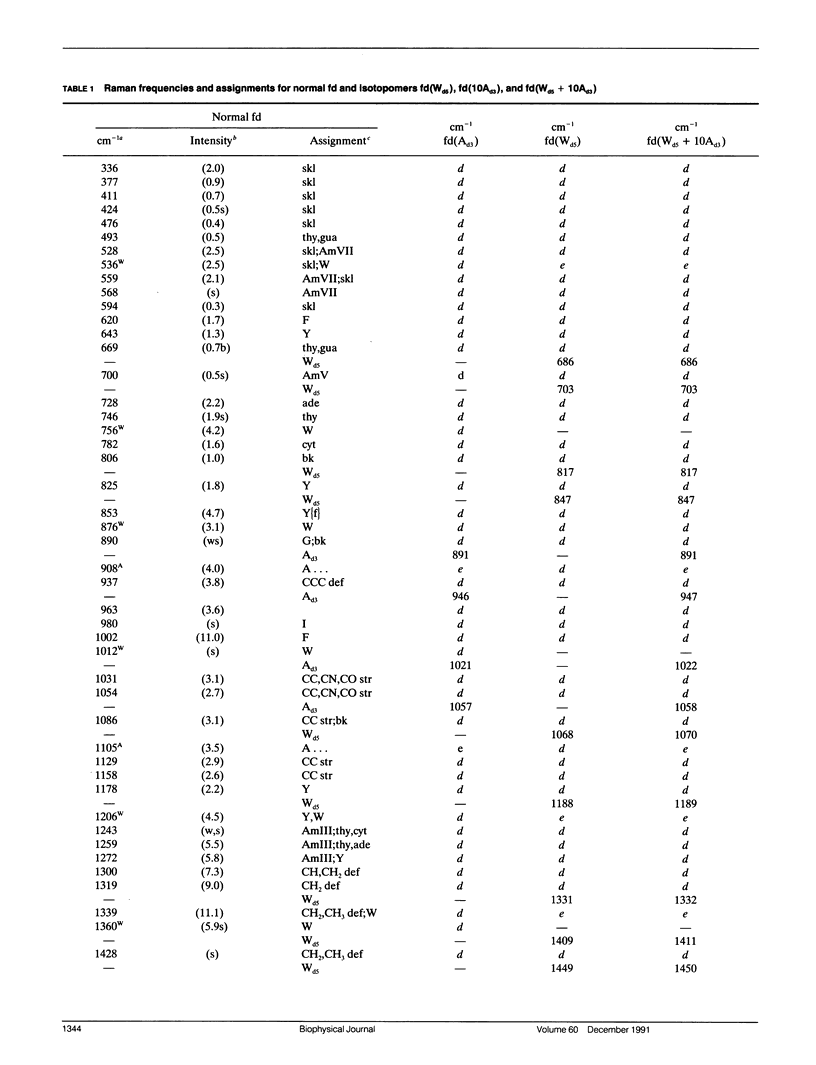
Abstract
Structural interpretation of the Raman spectra of filamentous bacteriophages is dependent upon reliable assignments for the numerous Raman vibrational bands contributed from coat protein and packaged DNA of the virion. To establish unambiguous assignments and facilitate structural conclusions derived from them, we have initiated a systematic study of filamentous bacteriophage Ff (fd, f1, M13) incorporating protein subunits with specifically deuterated amino-acid side chains. Here, we report and interpret the Raman spectra of fd virions which incorporate: (a) a single deuterio-tryptophan residue per coat protomer [fd(Wd5)], (b) ten deuterio-alanines per protomer [fd(10Ad3)], and (c) both deuterio-tryptophan and deuterio-alanine [fd(Wd5 + 10Ad3)]. The unambiguous assignment of coat protein Raman bands in normal and deuterated isotopomers of fd establishes the validity of earlier empirical assignments of many key Raman markers, including those of packaged ssDNA (Thomas et al., 1988). Present results confirm that deoxyguanosine residues of the packaged ssDNA molecule depart from the usual C2'-endo/anti conformation characteristic of protein-free DNA in aqueous solution, although C2'-endo/anti conformers of thymidine are not excluded by the data. The combined results obtained here on normal fd, and on fd incorporating deuterio-tryptophan [fd(Wd5) and fd(Wd5 + 10Ad3)], show also that the microenvironment of the single tryptophan residue per coat protomer (W26) can be clearly deduced as follows: (a) The indole 1-NH donor group of each protomer in fd forms a moderately strong hydrogen bond, most likely to a hydroxyl oxygen acceptor. (b) The planar indole ring exists in a hydrophilic environment. (c) The torsion angle describing the orientation of the indole ring (C3-C2 linkage) with respect to the side-chain (C alpha-C beta bond) is unusually large, i.e., magnitude of X2,1 approximately 120 degrees. With respect to alanine isotopomers, the present results show that alanine residues, and possibly other methyl-containing side chains, are significant contributors to the fd Raman spectrum. The present study provides new information on protomer side chains of fd and demonstrates a Raman methodology which should be generally useful for investigating single-site interactions and macromolecular conformations in other nucleoprotein assemblies.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benevides J. M., Stow P. L., Ilag L. L., Incardona N. L., Thomas G. J., Jr Differences in secondary structure between packaged and unpackaged single-stranded DNA of bacteriophage phi X174 determined by Raman spectroscopy: a model for phi X174 DNA packaging. Biochemistry. 1991 May 21;30(20):4855–4863. doi: 10.1021/bi00234a004. [DOI] [PubMed] [Google Scholar]
- Benevides J. M., Weiss M. A., Thomas G. J., Jr Design of the helix-turn-helix motif: nonlocal effects of quaternary structure in DNA recognition investigated by laser Raman spectroscopy. Biochemistry. 1991 May 7;30(18):4381–4388. doi: 10.1021/bi00232a003. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Raman spectra and vibrational assignments for deuterated membrane lipids. 1,2-Dipalmitoyl phosphatidylcholine-d9 and -d62. Biochim Biophys Acta. 1977 Nov 24;489(2):191–206. doi: 10.1016/0005-2760(77)90138-2. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Makowski L. The symmetries of filamentous phage particles. J Mol Biol. 1981 Jan 25;145(3):611–617. doi: 10.1016/0022-2836(81)90549-0. [DOI] [PubMed] [Google Scholar]
- Cross T. A., Tsang P., Opella S. J. Comparison of protein and deoxyribonucleic acid backbone structures in fd and Pf1 bacteriophages. Biochemistry. 1983 Feb 15;22(4):721–726. doi: 10.1021/bi00273a002. [DOI] [PubMed] [Google Scholar]
- Day L. A., Casadevall A., Prescott B., Thomas G. J., Jr Raman spectroscopy of mercury (II) binding to two filamentous viruses: Ff (fd, M13, f1) and Pf1. Biochemistry. 1988 Jan 26;27(2):706–711. doi: 10.1021/bi00402a032. [DOI] [PubMed] [Google Scholar]
- Day L. A., Marzec C. J., Reisberg S. A., Casadevall A. DNA packing in filamentous bacteriophages. Annu Rev Biophys Biophys Chem. 1988;17:509–539. doi: 10.1146/annurev.bb.17.060188.002453. [DOI] [PubMed] [Google Scholar]
- Day L. A., Wiseman R. L., Marzec C. J. Structure models for DNA in filamentous viruses with phosphates near the center. Nucleic Acids Res. 1979 Nov 24;7(6):1393–1403. doi: 10.1093/nar/7.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira M., Nishimura Y., Tsuboi M., Sato T., Mitsui Y., Iitaka Y. Local and overall conformations of DNA double helices with the A - T base pairs. Biochim Biophys Acta. 1986 Aug 22;867(4):256–267. doi: 10.1016/0167-4781(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Rohrer J., Gallusser A. Bacteriophages M13 and Pf3 tell us how proteins insert into the membrane. J Struct Biol. 1990 Jul-Sep;104(1-3):38–43. doi: 10.1016/1047-8477(90)90055-h. [DOI] [PubMed] [Google Scholar]
- Li Y., Thomas G. J., Jr, Fuller M., King J. Investigations of bacteriophage P22 by laser Raman spectroscopy. Prog Clin Biol Res. 1981;64:271–283. [PubMed] [Google Scholar]
- Marvin D. A. Dynamics of telescoping Inovirus: a mechanism for assembly at membrane adhesions. Int J Biol Macromol. 1989 Jun;11(3):159–164. doi: 10.1016/0141-8130(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Marvin D. A. Model-building studies of Inovirus: genetic variations on a geometric theme. Int J Biol Macromol. 1990 Apr;12(2):125–138. doi: 10.1016/0141-8130(90)90064-h. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Pigram W. J., Wiseman R. L., Wachtel E. J., Marvin F. J. Filamentous bacterial viruses. XIL. Molecular architecture of the class I (fd, If1, IKe) virion. J Mol Biol. 1974 Sep 25;88(3):581–598. doi: 10.1016/0022-2836(74)90409-4. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Wiseman R. L., Wachtel E. J. Filamentous bacterial viruses. XI. Molecular architecture of the class II (Pf1, Xf) virion. J Mol Biol. 1974 Jan 15;82(2):121–138. doi: 10.1016/0022-2836(74)90336-2. [DOI] [PubMed] [Google Scholar]
- O'Leary T. J., Levin I. W. Raman spectroscopy of selectively deuterated dimyristoylphosphatidylcholine: studies on dimyristoylphosphatidylcholine-cholesterol bilayers. Biochim Biophys Acta. 1986 Jan 29;854(2):321–324. doi: 10.1016/0005-2736(86)90126-4. [DOI] [PubMed] [Google Scholar]
- Opella S. J., Stewart P. L., Valentine K. G. Protein structure by solid-state NMR spectroscopy. Q Rev Biophys. 1987 Feb;19(1-2):7–49. doi: 10.1017/s0033583500004017. [DOI] [PubMed] [Google Scholar]
- Patapoff T. W., Thomas G. A., Wang Y., Peticolas W. L. Polarized Raman scattering from oriented single microcrystals of d(A5T5)2 and d(pTpT). Biopolymers. 1988 Mar;27(3):493–507. doi: 10.1002/bip.360270310. [DOI] [PubMed] [Google Scholar]
- Qian W., Bandekar J., Krimm S. Vibrational analysis of crystalline tri-L-alanine. Biopolymers. 1991 Feb 5;31(2):193–210. doi: 10.1002/bip.360310208. [DOI] [PubMed] [Google Scholar]
- Siamwiza M. N., Lord R. C., Chen M. C., Takamatsu T., Harada I., Matsuura H., Shimanouchi T. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry. 1975 Nov 4;14(22):4870–4876. doi: 10.1021/bi00693a014. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Benevides J. M. An A-helix structure for poly(dA-dT) X poly(dA-dT). Biopolymers. 1985 Jun;24(6):1101–1105. doi: 10.1002/bip.360240613. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Day L. A. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1 and Xf. Investigation by laser Raman spectroscopy. J Mol Biol. 1983 Apr 5;165(2):321–356. doi: 10.1016/s0022-2836(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Opella S. J., Day L. A. Sugar pucker and phosphodiester conformations in viral genomes of filamentous bacteriophages: fd, If1, IKe, Pf1, Xf, and Pf3. Biochemistry. 1988 Jun 14;27(12):4350–4357. doi: 10.1021/bi00412a023. [DOI] [PubMed] [Google Scholar]
- Thomas G., Jr, Murphy P. Structure of coat proteins in Pf1 and fd virions by laser raman spectroscopy. Science. 1975 Jun 20;188(4194):1205–1207. doi: 10.1126/science.1170637. [DOI] [PubMed] [Google Scholar]


