Abstract
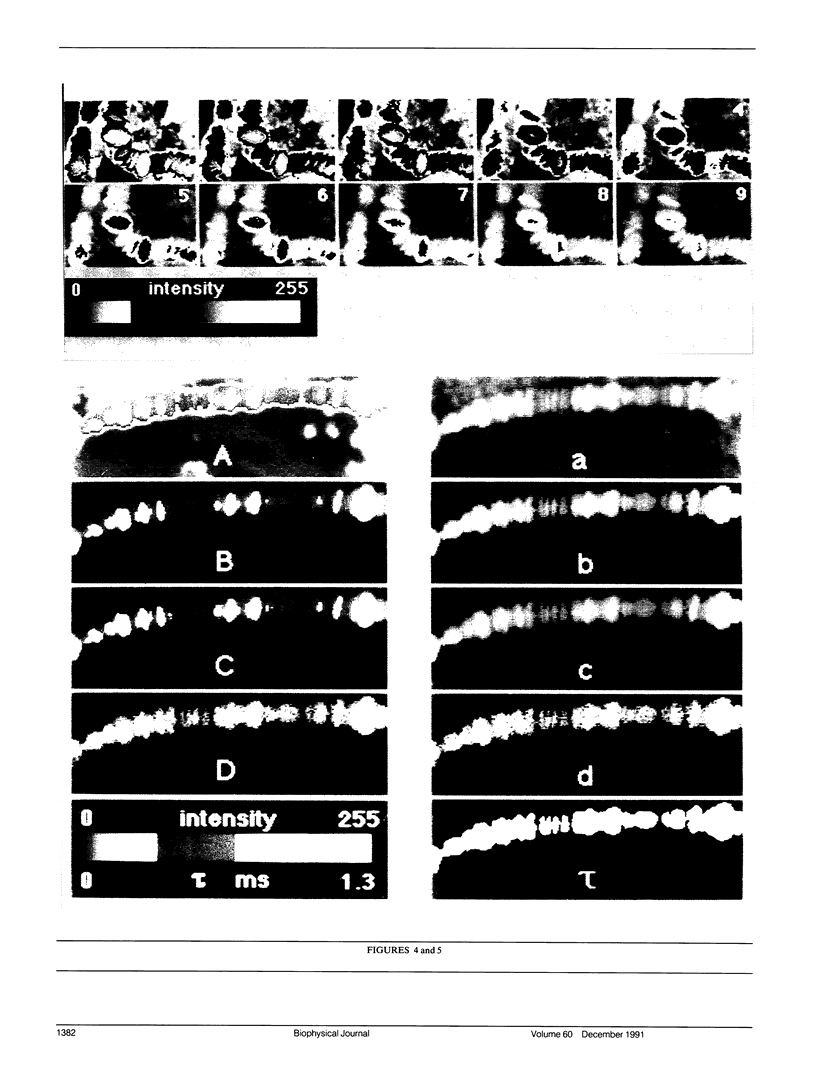
An optical microscope capable of measuring time resolved luminescence (phosphorescence and delayed fluorescence) images has been developed. The technique employs two phase-locked mechanical choppers and a slow-scan scientific CCD camera attached to a normal fluorescence microscope. The sample is illuminated by a periodic train of light pulses and the image is recorded within a defined time interval after the end of each excitation period. The time resolution discriminates completely against light scattering, reflection, autofluorescence, and extraneous prompt fluorescence, which ordinarily decrease contrast in normal fluorescence microscopy measurements. Time resolved image microscopy produces a high contrast image and particular structures can be emphasized by displaying a new parameter, the ratio of the phosphorescence to fluorescence. Objects differing in luminescence decay rates are easily resolved. The lifetime of the long lived luminescence can be measured at each pixel of the microscope image by analyzing a series of images that differ by a variable time delay. The distribution of luminescence decay rates is displayed directly as an image. Several examples demonstrate the utility of the instrument and the complementarity it offers to conventional fluorescence microscopy.
Full text
PDF

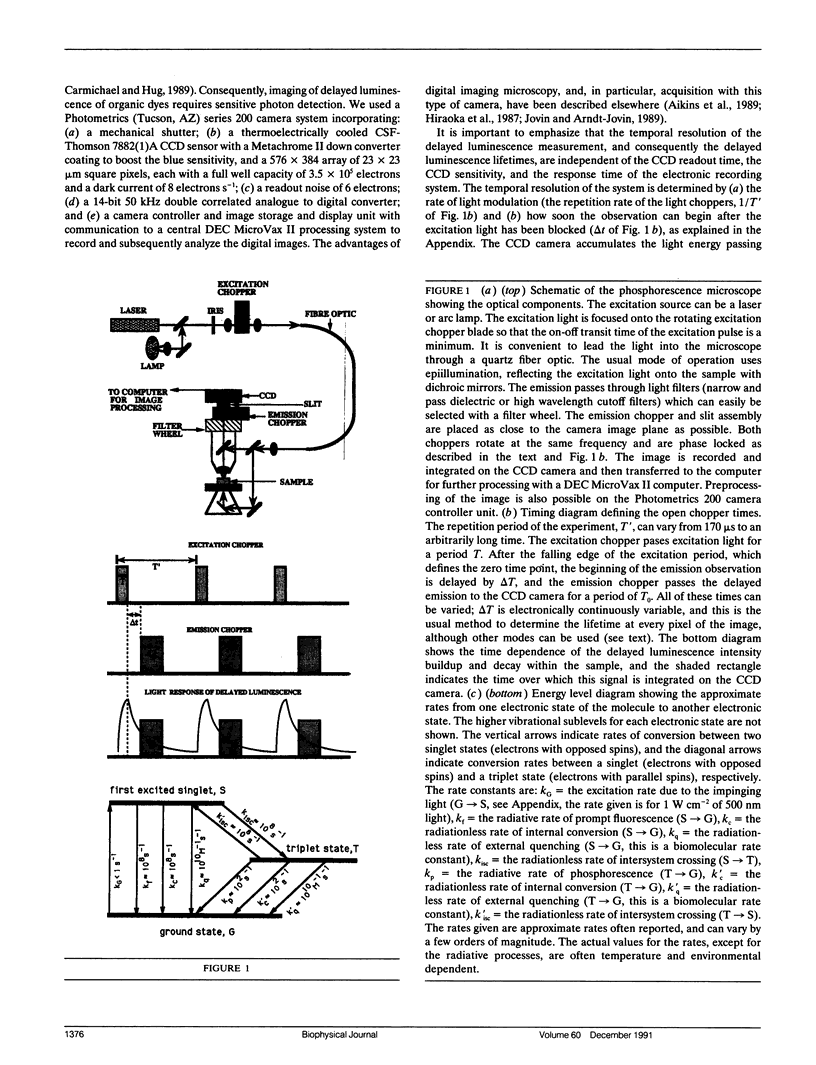









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikens R. S., Agard D. A., Sedat J. W. Solid-state imagers for microscopy. Methods Cell Biol. 1989;29:291–313. doi: 10.1016/s0091-679x(08)60199-5. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Robert-Nicoud M., Kaufman S. J., Jovin T. M. Fluorescence digital imaging microscopy in cell biology. Science. 1985 Oct 18;230(4723):247–256. doi: 10.1126/science.4048934. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Chan S. S., Jovin T. M. Rotational diffusion of cell surface components by time-resolved phosphorescence anisotropy. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5650–5654. doi: 10.1073/pnas.76.11.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverloo H. B., van Schadewijk A., van Gelderen-Boele S., Tanke H. J. Inorganic phosphors as new luminescent labels for immunocytochemistry and time-resolved microscopy. Cytometry. 1990;11(7):784–792. doi: 10.1002/cyto.990110704. [DOI] [PubMed] [Google Scholar]
- Corin A. F., Jovin T. M. Proflavin binding to poly[d(A-T)] and poly[d(A-br5U)]: triplet state and temperature-jump kinetics. Biochemistry. 1986 Jul 15;25(14):3995–4007. doi: 10.1021/bi00362a004. [DOI] [PubMed] [Google Scholar]
- DeBiasio R., Bright G. R., Ernst L. A., Waggoner A. S., Taylor D. L. Five-parameter fluorescence imaging: wound healing of living Swiss 3T3 cells. J Cell Biol. 1987 Oct;105(4):1613–1622. doi: 10.1083/jcb.105.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
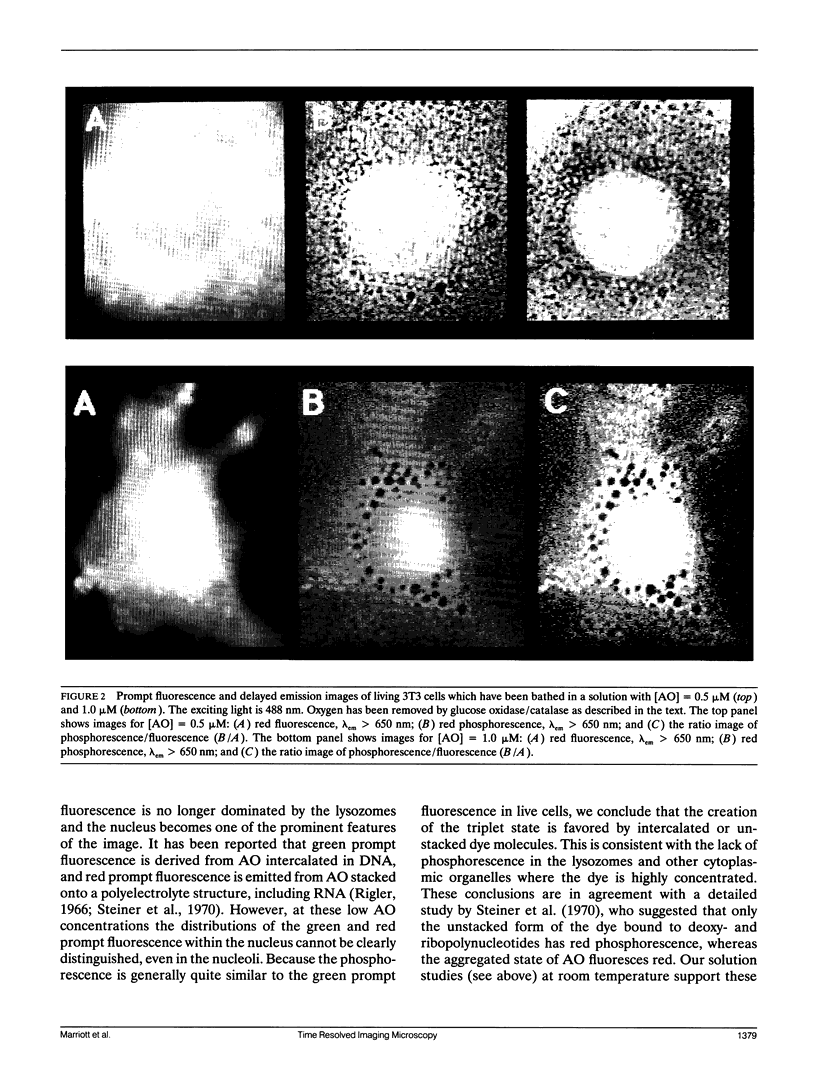
- Delic J., Coppey J., Magdelenat H., Coppey-Moisan M. Impossibility of acridine orange intercalation in nuclear DNA of the living cell. Exp Cell Res. 1991 May;194(1):147–153. doi: 10.1016/0014-4827(91)90144-j. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J. Biochemistry without oxygen. Anal Biochem. 1987 Mar;161(2):300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Moore C. H. Phosphorescence of protein-bound eosin and erythrosin. A possible probe for measurements of slow rotational mobility. Biochem J. 1979 Dec 1;183(3):561–572. doi: 10.1042/bj1830561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmilä I., Dakubu S., Mukkala V. M., Siitari H., Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984 Mar;137(2):335–343. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987 Oct 2;238(4823):36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- Ho W. C., Allan V. J., van Meer G., Berger E. G., Kreis T. E. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989 Apr;48(2):250–263. [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Depolarization of fluorescence depletion. A microscopic method for measuring rotational diffusion of membrane proteins on the surface of a single cell. FEBS Lett. 1981 Sep 28;132(2):252–256. doi: 10.1016/0014-5793(81)81172-6. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Arndt-Jovin D. J. Luminescence digital imaging microscopy. Annu Rev Biophys Biophys Chem. 1989;18:271–308. doi: 10.1146/annurev.bb.18.060189.001415. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Vaz W. L. Rotational and translational diffusion in membranes measured by fluorescence and phosphorescence methods. Methods Enzymol. 1989;172:471–513. doi: 10.1016/s0076-6879(89)72030-9. [DOI] [PubMed] [Google Scholar]
- Lipsky N. G., Pagano R. E. A vital stain for the Golgi apparatus. Science. 1985 May 10;228(4700):745–747. doi: 10.1126/science.2581316. [DOI] [PubMed] [Google Scholar]
- Poliakov Ia S., Rozanov Iu M., Brumberg E. M. Ustanovka dlia issledovaniia fosforestsentsii mikroob"ektov. Tsitologiia. 1966 Sep-Oct;8(5):677–681. [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. I. INTERRELATIONSHIPS OF ACRIDINE ORANGE PARTICLES AND CYTOPLASMIC REDDENING. J Cell Biol. 1963 Aug;18:237–250. doi: 10.1083/jcb.18.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., MARCUS P. I., GONATAS N. K. DYNAMICS OF ACRIDINE ORANGE-CELL INTERACTION. II. DYE-INDUCED ULTRASTRUCTURAL CHANGES IN MULTIVESICULAR BODIES (ACRIDINE ORANGE PARTICLES). J Cell Biol. 1964 Apr;21:49–62. doi: 10.1083/jcb.21.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey W. L., Vanderkooi J. M., Wilson D. F. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988 Sep 23;241(4873):1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- Steiner R. F., Weinryb I., Kolinski R. The low temperature luminescence of complexes of acridine orange with polyelectrolytes. Biochim Biophys Acta. 1970;209(2):306–319. doi: 10.1016/0005-2787(70)90729-x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent indicators of ion concentrations. Methods Cell Biol. 1989;30:127–156. doi: 10.1016/s0091-679x(08)60978-4. [DOI] [PubMed] [Google Scholar]
- VIALLI M. INDIRIZZI DI RICERCA NEL CAMPO DELLA ISTOLUMINESCENZA. Z Wiss Mikrosk. 1964 Mar;66:164–170. [PubMed] [Google Scholar]
- Vanderkooi J. M., Maniara G., Green T. J., Wilson D. F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987 Apr 25;262(12):5476–5482. [PubMed] [Google Scholar]
- Zelenin A. V. Fluorescence microscopy of lysosomes and related structures in living cells. Nature. 1966 Oct 22;212(5060):425–426. doi: 10.1038/212425a0. [DOI] [PubMed] [Google Scholar]
- Zotikov A. A., Polyakov Y. S. The use of the phosphorescence microscope for the study of the phosphorescence of various cells. Microsc Acta. 1977 Sep;79(5):415–418. [PubMed] [Google Scholar]