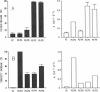
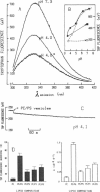
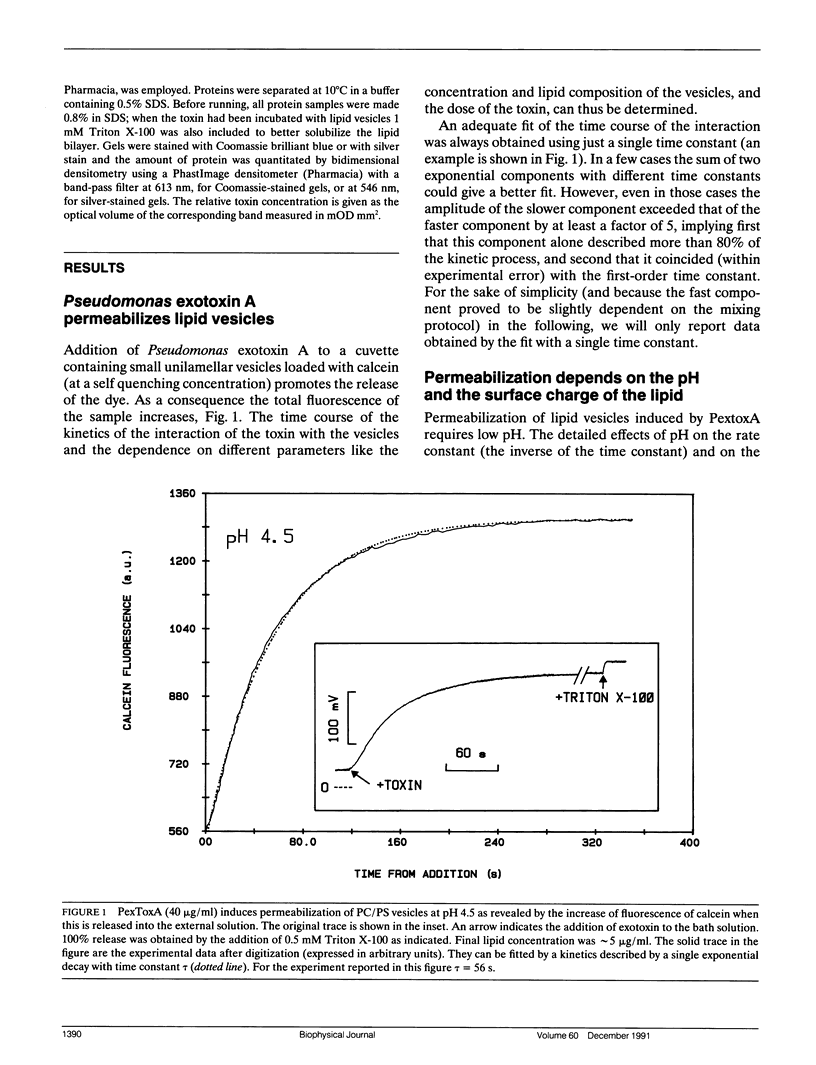
Abstract
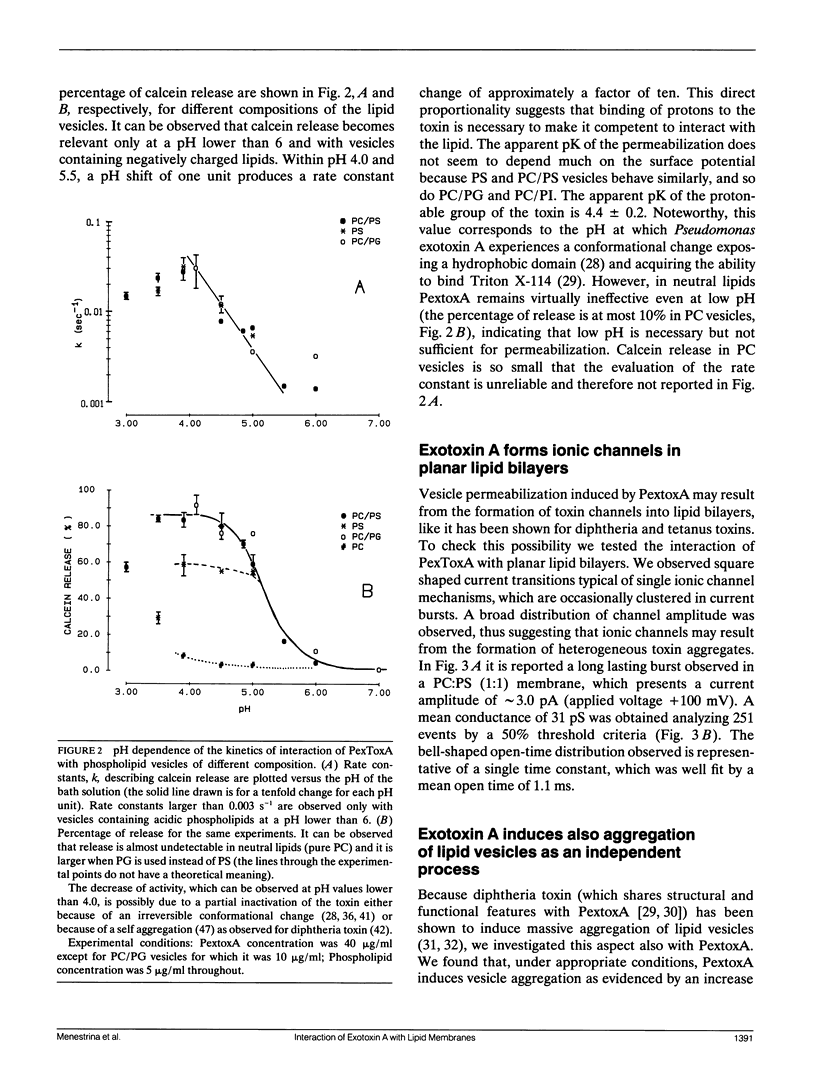
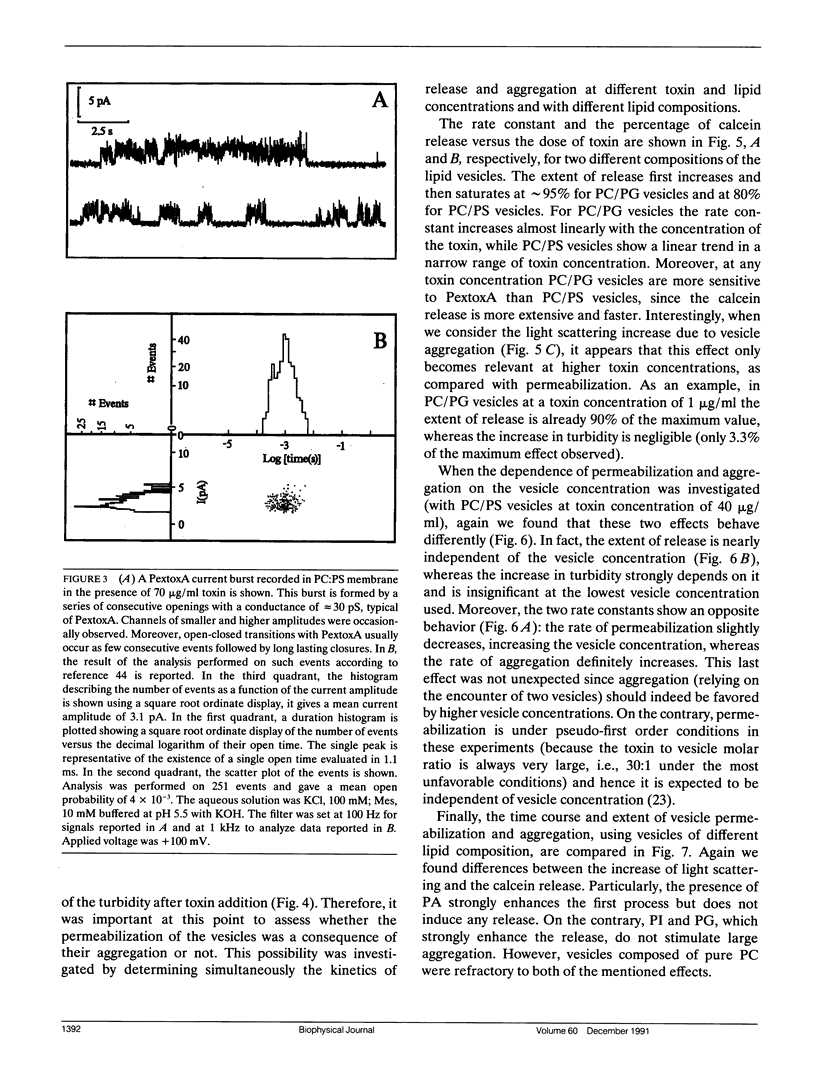
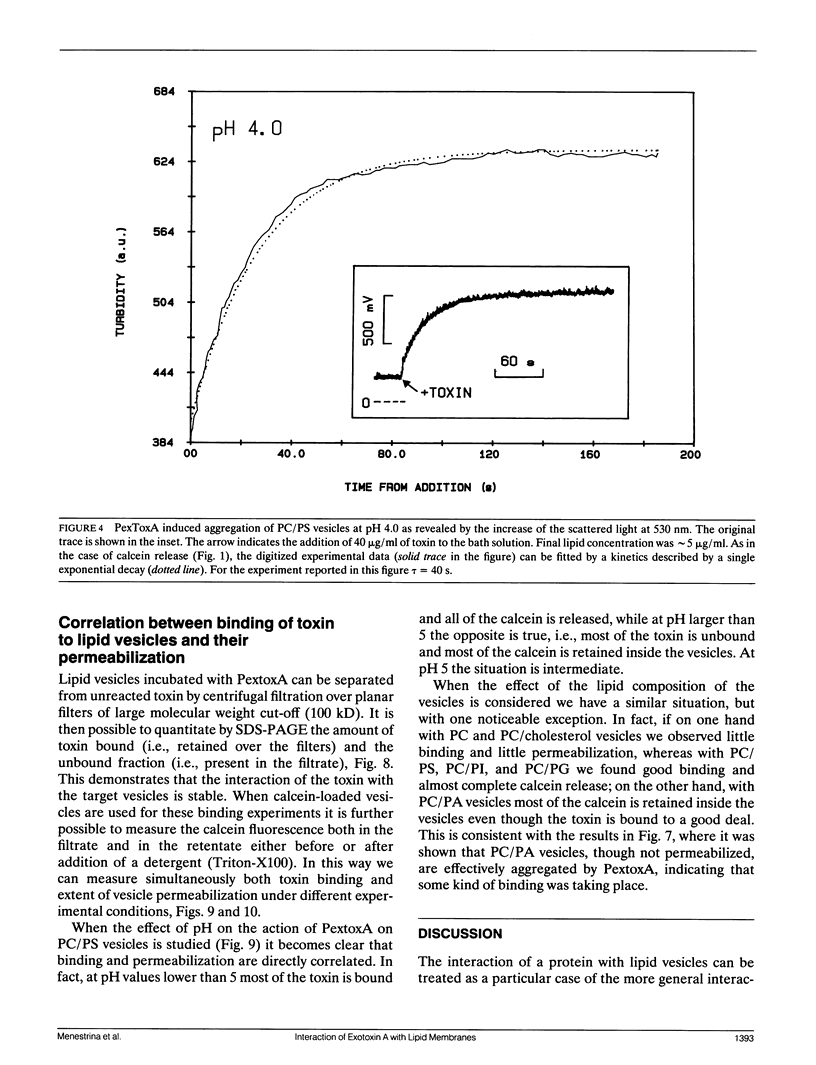
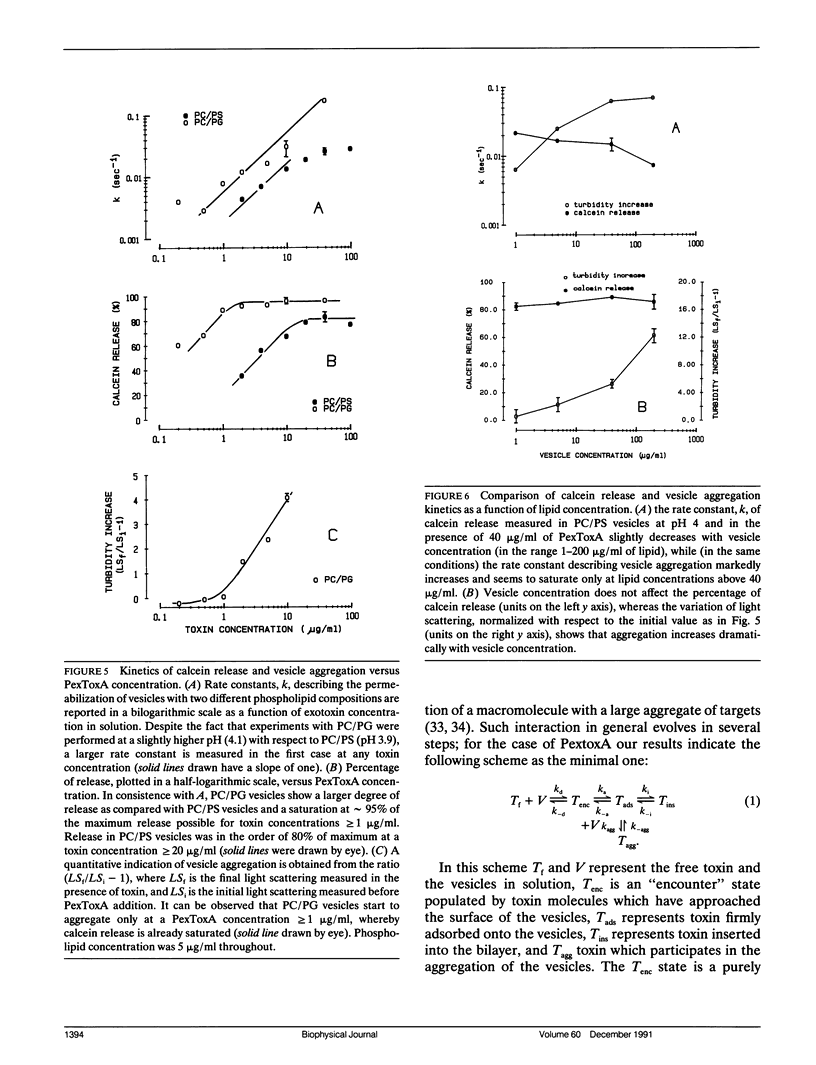
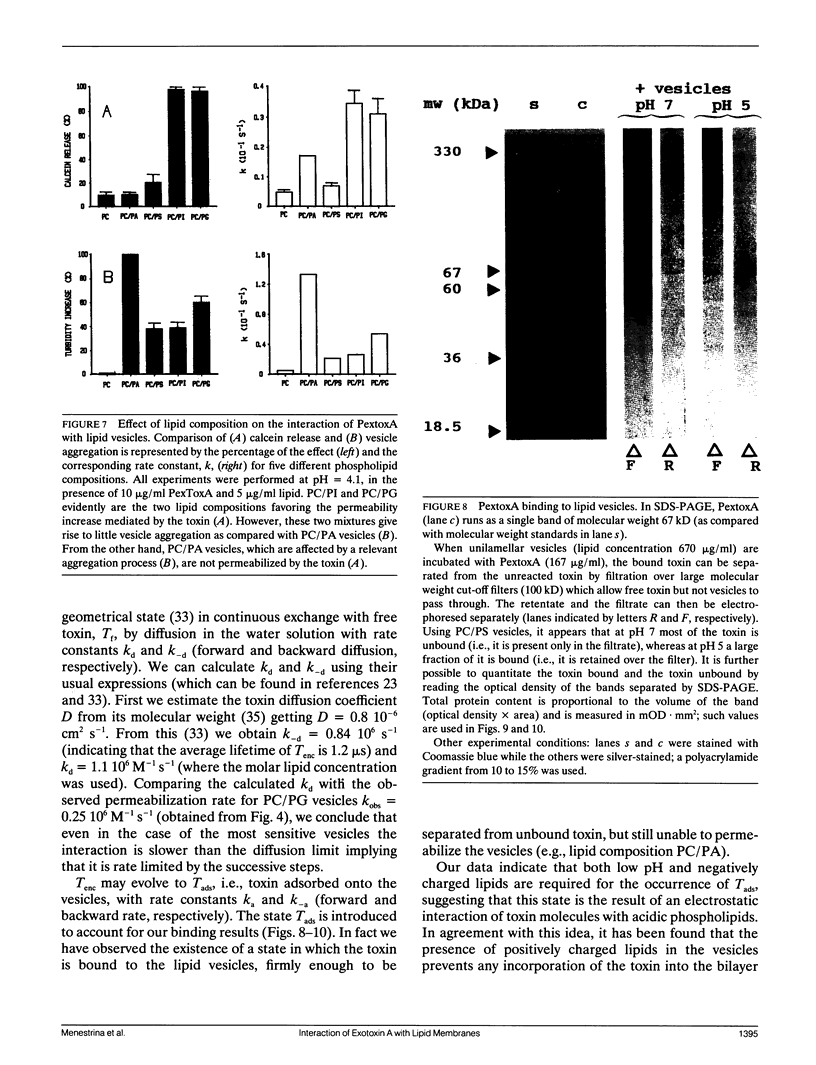
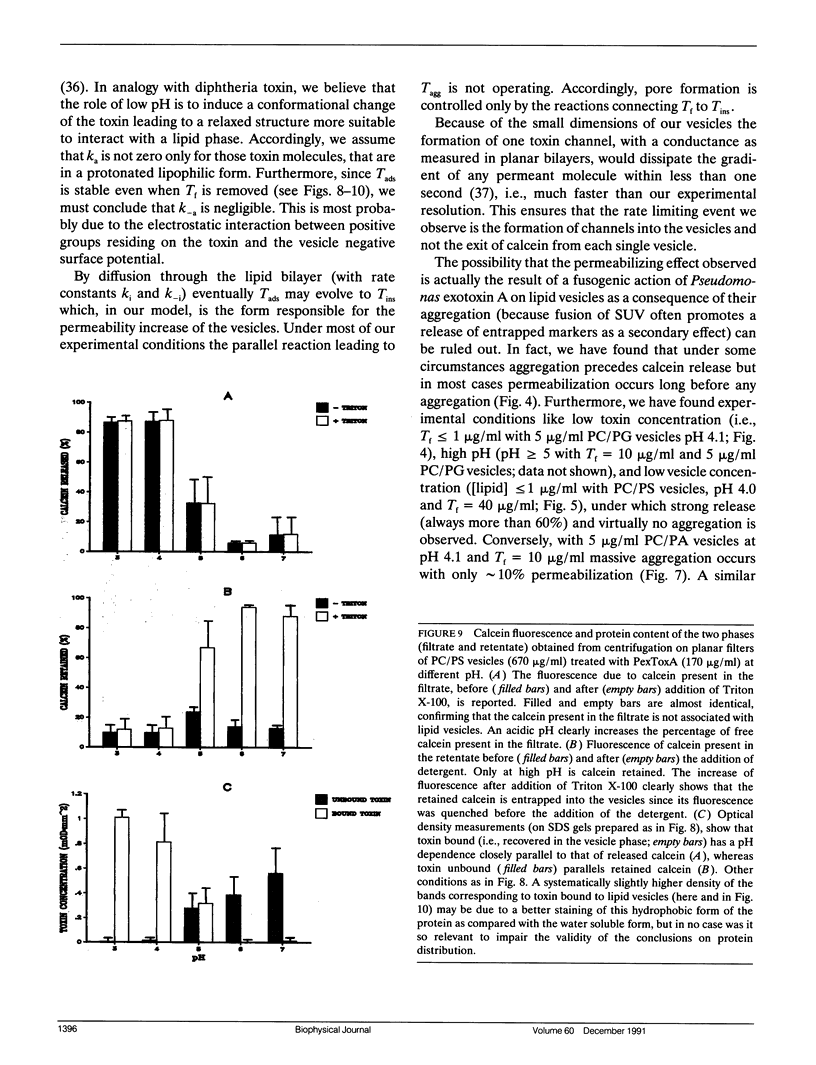
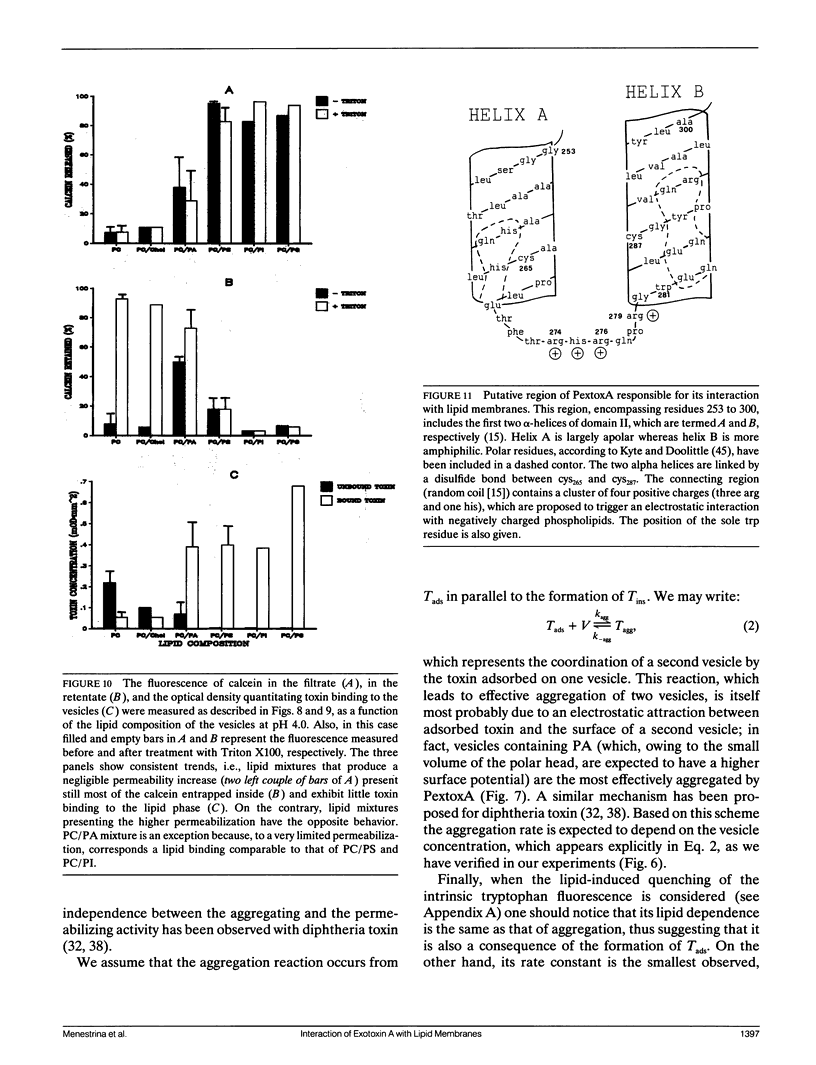
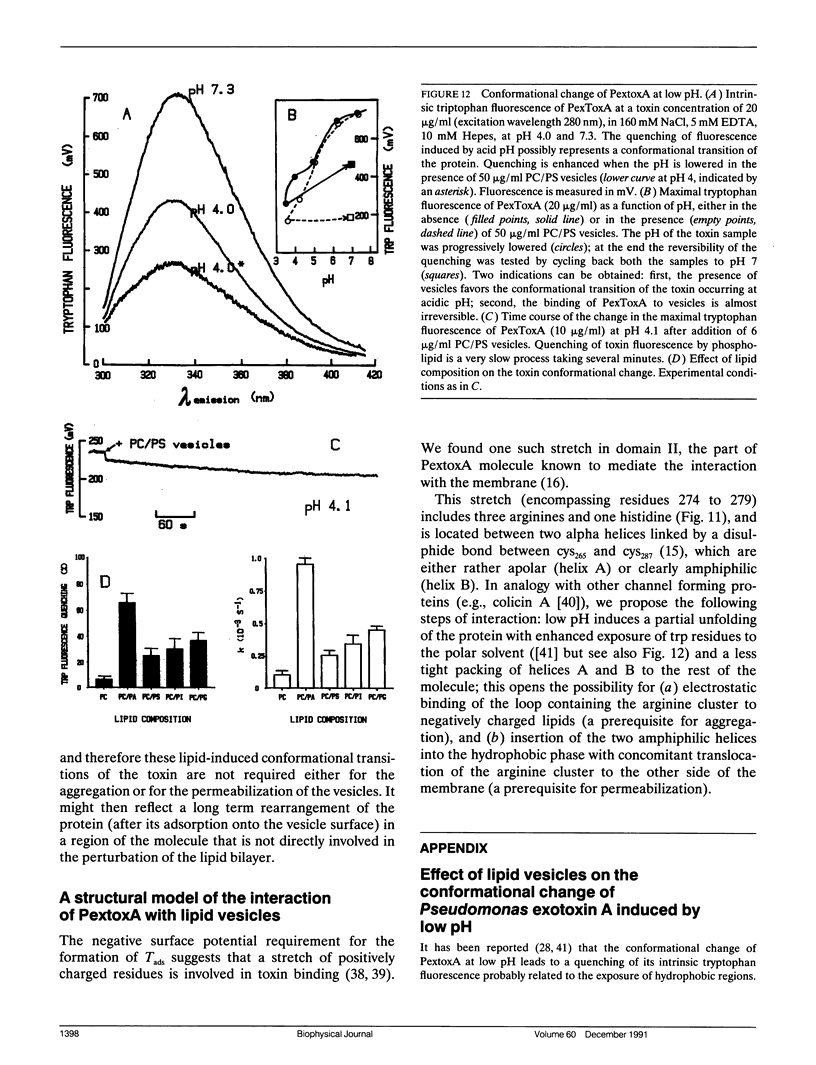
We have investigated the interaction of Pseudomonas exotoxin A with small unilamellar vesicles comprised of different phospholipids as a function of pH, toxin, and lipid concentration. We have found that this toxin induces vesicle permeabilization, as measured by the release of a fluorescent dye. Permeabilization is due to the formation of ion-conductive channels which we have directly observed in planar lipid bilayers. The toxin also produces vesicle aggregation, as indicated by an increase of the turbidity. Aggregation and permeabilization have completely different time course and extent upon toxin dose and lipid composition, thus suggesting that they are two independent events. Both time constants decrease by lowering the pH of the bulk phase or by introducing a negative lipid into the vesicles. Our results indicate that at least three steps are involved in the interaction of Pseudomonas exotoxin A with lipid vesicles. After protonation of one charged group the toxin becomes competent to bind to the surface of the vesicles. Binding is probably initiated by an electrostatic interaction because it is absolutely dependent on the presence of acidic phospholipids. Binding is a prerequisite for the subsequent insertion of the toxin into the lipid bilayer, with a special preference for phosphatidylglycerol-containing membranes, to form ionic channels. At high toxin and vesicle concentrations, bound toxin may also induce aggregation of the vesicles, particularly when phosphatidic acid is present in the lipid mixture. A quenching of the intrinsic tryptophan fluorescence of the protein, which is induced by lowering the pH of the solution, becomes more drastic in the presence of lipid vesicles. However, this further quenching takes so long that it cannot be a prerequisite to either vesicle permeabilization or aggregation. Pseudomonas exotoxin A shares many of these properties with other bacterial toxins like diphtheria and tetanus toxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Blewitt M. G., Chung L. A., London E. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry. 1985 Sep 24;24(20):5458–5464. doi: 10.1021/bi00341a027. [DOI] [PubMed] [Google Scholar]
- Cabiaux V., Vandenbranden M., Falmagne P., Ruysschaert J. M. Diphtheria toxin induces fusion of small unilamellar vesicles at low pH. Biochim Biophys Acta. 1984 Aug 8;775(1):31–36. doi: 10.1016/0005-2736(84)90231-1. [DOI] [PubMed] [Google Scholar]
- Defrise-Quertain F., Cabiaux V., Vandenbranden M., Wattiez R., Falmagne P., Ruysschaert J. M. pH-dependent bilayer destabilization and fusion of phospholipidic large unilamellar vesicles induced by diphtheria toxin and its fragments A and B. Biochemistry. 1989 Apr 18;28(8):3406–3413. doi: 10.1021/bi00434a040. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Baldwin R. L., Wisnieski B. J. Effect of low pH on the conformation of Pseudomonas exotoxin A. J Biol Chem. 1987 Feb 15;262(5):2256–2261. [PubMed] [Google Scholar]
- Farahbakhsh Z. T., Baldwin R. L., Wisnieski B. J. Pseudomonas exotoxin A. Membrane binding, insertion, and traversal. J Biol Chem. 1986 Aug 25;261(24):11404–11408. [PubMed] [Google Scholar]
- Forti S., Menestrina G. Staphylococcal alpha-toxin increases the permeability of lipid vesicles by cholesterol- and pH-dependent assembly of oligomeric channels. Eur J Biochem. 1989 May 15;181(3):767–773. doi: 10.1111/j.1432-1033.1989.tb14790.x. [DOI] [PubMed] [Google Scholar]
- Gambale F., Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys J. 1988 May;53(5):771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Chen M. S. Structure and function relationship of Pseudomonas exotoxin A. An immunochemical study. J Biol Chem. 1989 Feb 5;264(4):2379–2384. [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Jiang J. X., London E. Involvement of denaturation-like changes in Pseudomonas exotoxin a hydrophobicity and membrane penetration determined by characterization of pH and thermal transitions. Roles of two distinct conformationally altered states. J Biol Chem. 1990 May 25;265(15):8636–8641. [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Forti S., Gambale F. Interaction of tetanus toxin with lipid vesicles. Effects of pH, surface charge, and transmembrane potential on the kinetics of channel formation. Biophys J. 1989 Mar;55(3):393–405. doi: 10.1016/S0006-3495(89)82833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S. Gating of ion channels made by a diphtheria toxin fragment in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4320–4324. doi: 10.1073/pnas.80.14.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Moskaug J. O., Stenmark H., Sandvig K. Diphtheria toxin entry: protein translocation in the reverse direction. Trends Biochem Sci. 1988 Sep;13(9):348–351. doi: 10.1016/0968-0004(88)90105-3. [DOI] [PubMed] [Google Scholar]
- Papini E., Colonna R., Cusinato F., Montecucco C., Tomasi M., Rappuoli R. Lipid interaction of diphtheria toxin and mutants with altered fragment B. 1. Liposome aggregation and fusion. Eur J Biochem. 1987 Dec 15;169(3):629–635. doi: 10.1111/j.1432-1033.1987.tb13654.x. [DOI] [PubMed] [Google Scholar]
- Papini E., Sandoná D., Rappuoli R., Montecucco C. On the membrane translocation of diphtheria toxin: at low pH the toxin induces ion channels on cells. EMBO J. 1988 Nov;7(11):3353–3359. doi: 10.1002/j.1460-2075.1988.tb03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Pattus F., Tucker A. D., Tsernoglou D. Structure of the membrane-pore-forming fragment of colicin A. Nature. 1989 Jan 5;337(6202):93–96. doi: 10.1038/337093a0. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Tucker A. D., Tsernoglou D., Pattus F. Insights into membrane insertion based on studies of colicins. Trends Biochem Sci. 1990 Apr;15(4):126–129. doi: 10.1016/0968-0004(90)90205-p. [DOI] [PubMed] [Google Scholar]
- Pederzolli C., Cescatti L., Menestrina G. Chemical modification of Staphylococcus aureus alpha-toxin by diethylpyrocarbonate: role of histidines in its membrane-damaging properties. J Membr Biol. 1991 Jan;119(1):41–52. doi: 10.1007/BF01868539. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Small G. J., Warren H. B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990 Aug 3;249(4968):537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Moskaug J. O. Pseudomonas toxin binds triton X-114 at low pH. Biochem J. 1987 Aug 1;245(3):899–901. doi: 10.1042/bj2450899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J Biol Chem. 1988 Sep 5;263(25):12352–12359. [PubMed] [Google Scholar]
- Schwarz G. Basic kinetics of binding and incorporation with supramolecular aggregates. Biophys Chem. 1987 May 9;26(2-3):163–169. doi: 10.1016/0301-4622(87)80019-4. [DOI] [PubMed] [Google Scholar]
- Schwarz G., Gerke H., Rizzo V., Stankowski S. Incorporation kinetics in a membrane, studied with the pore-forming peptide alamethicin. Biophys J. 1987 Nov;52(5):685–692. doi: 10.1016/S0006-3495(87)83263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver J. W., Donovan J. J. Interactions of diphtheria toxin with lipid vesicles: determinants of ion channel formation. Biochim Biophys Acta. 1987 Sep 18;903(1):48–55. doi: 10.1016/0005-2736(87)90154-4. [DOI] [PubMed] [Google Scholar]
- Siegall C. B., Chaudhary V. K., FitzGerald D. J., Pastan I. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J Biol Chem. 1989 Aug 25;264(24):14256–14261. [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. The clinical challenge of infections due to Pseudomonas aeruginosa. Rev Infect Dis. 1984 Sep-Oct;6 (Suppl 3):S603–S607. doi: 10.1093/clinids/6.supplement_3.s603. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Characterization of the insertion of Pseudomonas exotoxin A into membranes. Infect Immun. 1985 Dec;50(3):630–635. doi: 10.1128/iai.50.3.630-635.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Mechanism of insertion of diphtheria toxin: peptide entry and pore size determinations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3341–3345. doi: 10.1073/pnas.81.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. M., London E. Conformation and model membrane interactions of diphtheria toxin fragment A. J Biol Chem. 1988 Oct 25;263(30):15369–15377. [PubMed] [Google Scholar]
- Zhao J. M., London E. Localization of the active site of diphtheria toxin. Biochemistry. 1988 May 3;27(9):3398–3403. doi: 10.1021/bi00409a041. [DOI] [PubMed] [Google Scholar]