Abstract
The preprotein translocation at the inner envelope membrane of chloroplasts so far involves five proteins: Tic110, Tic55, Tic40, Tic22 and Tic20. The molecular function of these proteins has not yet been established. Here, we demonstrate that Tic110 constitutes a central part of the preprotein translocation pore. Dependent on the presence of intact Tic110, radiolabelled preprotein specifically interacts with isolated inner envelope vesicles as well as with purified, recombinant Tic110 reconstituted into liposomes. Circular dichroism analysis reveals that Tic110 consists mainly of β-sheets, a structure typically found in pore proteins. In planar lipid bilayers, recombinant Tic110 forms a cation-selective high-conductance channel with a calculated inner pore opening of 1.7 nm. Purified transit peptide causes strong flickering and a voltage-dependent block of the channel. Moreover, at the inner envelope membrane, a peptide-sensitive channel is described that shows properties basically identical to the channel formed by recombinant Tic110. We conclude that Tic110 has a distinct preprotein binding site and functions as a preprotein translocation pore at the inner envelope membrane.
Keywords: chloroplast/import/inner envelope/reconstitution/Tic complex
Introduction
The biogenesis and function of photosynthetically active chloroplasts requires the transport of nuclear encoded preproteins across the envelope membranes. Protein complexes localized at the outer (Toc complex) and the inner (Tic complex) envelope membrane accomplish the translocation of preproteins, which are routed to the organelle’s surface by a cleavable N-terminal transit peptide. At the envelope membranes, the uptake of preproteins into the chloroplast comprises three fundamental steps. The initial docking and recognition process seems to be independent of ATP hydrolysis (Perry and Keegstra, 1994). The participating receptor proteins Toc34 and Toc159 are nucleotide-binding proteins and furthermore, their functioning is probably dependent on nucleoside triphosphates (Kouranov and Schnell, 1997; Sveshnikova et al., 2000). Next, the translocation pore Toc75 interacts with the preprotein (Hinnah et al., 1997) that is finally conducted through joint translocation sites consisting of both the Toc and the Tic complex (Caliebe et al., 1997; Kouranov and Schnell, 1997; Nielsen et al., 1997). Though the Tic complex of chloroplasts was expected to fit to the current concept of protein translocation across membranes (Schatz and Dobberstein, 1996), an individual function such as a receptor or a translocation pore could not be assigned to the known Tic components Tic110, Tic55, Tic40, Tic22 and Tic20 (Kessler and Blobel, 1996; Lübeck et al., 1996; Caliebe et al., 1997; Kouranov et al., 1998; Stahl et al., 1999). As shown by label-transfer cross-linking experiments, Tic20 and Tic22 are close to a chimeric preprotein. Since Tic22 forms a cross-link product with preprotein, which was not engaged to the Tic complex, a function as a chaperone was assumed. Tic20 is deeply embedded in the inner envelope membrane and therefore is proposed to function as a protein conducting component of the Tic complex (Kouranov and Schnell, 1997; Kouranov et al., 1998). However, experimental evidence supporting this notion was not provided. Tic40 was initially localized at both the outer and inner envelope membrane (Wu et al., 1994; Ko et al., 1995), but later it was predominantly found at the inner envelope membrane (Stahl et al., 1999). Complementation of the SecA defect of an Escherichia coli mutant (Pang et al., 1997) and the similarity of its C-terminus to Hsp70-binding proteins lead to the assumption that Tic40 might recruit molecular chaperones (Stahl et al., 1999). The Rieske-type iron-sulfur protein Tic55 might function as a regulator (Caliebe et al., 1997). While the affiliation with the Tic complex is argued for all Tic proteins mentioned previously, Tic110 is the only generally accepted component. Using cross-link experiments, co-immunoprecipitation and affinity chromatography, Tic110 was found together with a translocating preprotein and components of the protein import apparatus at the envelope membranes (Kessler and Blobel, 1996; Lübeck et al., 1996; Caliebe et al., 1997; Nielsen et al., 1997; Kouranov et al., 1998; Stahl et al., 1999). Several functions of Tic110 are suggested. On the one hand, Tic110 might be involved in the formation of joint translocation sites of the Toc and the Tic complex, because Tic110 was found in a cross-link product together with Toc75 and a preprotein (Lübeck et al., 1996). On the other hand, Tic110 could act by recruiting stromal chaperones such as cpn60 and ClpC that have been described to form a cross-link product with Tic110 (Kessler and Blobel, 1996; Nielsen et al., 1997). Furthermore, a ‘protein import related anion channel’ (PIRAC) was found to be associated with Tic110. Using inside-out patches of both the outer and inner envelope membrane, an antiserum raised against Tic110 abolished PIRAC activity (van den Wijngaard and Vredenberg, 1999). With the preprotein or the transit peptide of ferredoxin A decreased open probability of PIRAC was observed (van den Wijngaard and Vredenberg, 1997). On the assumption that due to the abundance of large pores the outer envelope membrane was highly permeable, the authors concluded that the observed anion channel activity was localized at the inner envelope membrane, but the molecular identity of PIRAC remains elusive (van den Wijngaard and Vredenberg, 1999).
In summary, the role of Tic110 during preprotein translocation is not yet clear. In this paper, we show that both isolated inner envelope vesicles and proteoliposomes containing recombinant Tic110 are able to interact with preproteins dependent on the transit peptide. After fusion into planar lipid bilayers recombinant, Tic110 forms a cation-selective channel sensitive to transit peptide. We conclude that Tic110 forms the central preprotein translocation pore at the inner envelope membrane.
Results
Binding of a preprotein to isolated inner envelope vesicles
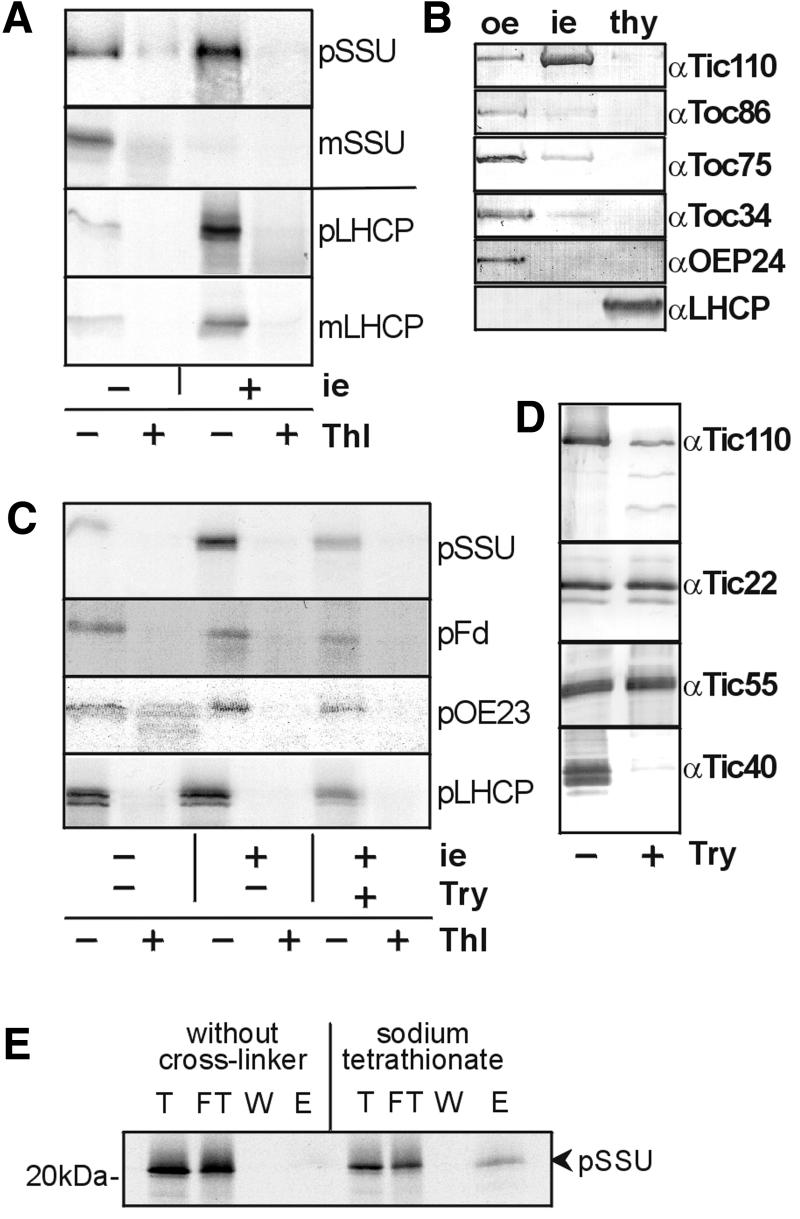
While the protein translocase of the inner membrane of mitochondria is accessible after osmotic disruption of the outer membrane (Segui-Real et al., 1993), a similar method has not been established for chloroplasts. However, isolated vesicles of the outer membrane from both chloroplasts (Waegemann and Soll, 1991) and mitochondria (Mayer et al., 1993) can be used as a bona fide system to study the initial steps of the binding of preproteins. Inner envelope vesicles are isolated mainly in a right-side-out orientation (L.Heins and J.Soll, unpublished data). Therefore, we attempted to investigate the capacity of isolated inner envelope vesicles to interact specifically with a preprotein. The 35S-labelled preprotein of the small subunit of ribulose bisphosphate carboxylase (pSSU) and the light harvesting complex protein II (pLHCP) bound to isolated vesicles at 25°C in the presence of 0.1% bovine serum albumin (BSA) and 1 mM ATP (Figure 1A). The interaction of their mature forms (mSSU and mLHCP, respectively) with inner envelope vesicles was significantly reduced (Figure 1A). These results indicate that the interaction is due to the transit peptide (Figure 1A). The hydrophobic character of mLHCP is probably responsible for a minor amount of mLHCP found to be associated with the inner envelope vesicles. All proteins used for the binding experiment remained highly sensitive towards treatment with thermolysin either with or without inner envelope vesicles, demonstrating that the translation products maintain a loosely folded state during binding. Therefore, pSSU and pLHCP are not re-isolated due to aggregation at the membrane surface, but due to the specific interaction of their transit peptide with the vesicle surface. The binding of preproteins to inner envelope vesicles probably did not result from a contamination with outer envelope vesicles or thylakoid membranes as shown by immunodecoration of marker proteins of the membrane fractions. Components of the preprotein translocon at the outer envelope membrane of chloroplasts, Toc86, Toc75 and Toc34, and LHCP localized at the thylakoids were hardly detectable at purified inner envelope vesicles (Figure 1B). Treatment of the inner envelope vesicles with trypsin prior to the addition of preprotein significantly decreased the binding of pSSU, pLHCP, the preproteins of ferredoxin and the 23 kDa protein of the oxygen-evolving complex (Figure 1C), indicating that this interaction is dependent on protein epitopes at the surface of the vesicles. Even though some preprotein was still detected in contact with the vesicles, the efficiency of binding dropped to 50% (on average of four experiments). The components of the Tic complex were affected in different ways by trypsin treatment of isolated vesicles (Figure 1D). While Tic22 and Tic55 remained intact, Tic40 was degraded completely. If binding of the preprotein mainly depends on Tic40, an interaction of the preprotein should no longer be observed with vesicles pre-treated with protease. Proteolytic treatment of Tic110 resulted in a specific proteolytic pattern, which has likewise been observed by Lübeck et al. (1996), and a small amount of Tic110 remained intact. However, several of the degradation products might have retained the preprotein-binding capacity. These results support the notion that not Tic40 but another, not yet identified Tic component or more probable Tic110 plays a major role in binding the preprotein. In proof of an interaction of Tic110 and a preprotein, 35S-labelled pSSU was bound first to purified isolated inner envelope vesicles and then the homobifunctional cross-linker sodium tetrathionate was added. After solubilization and immunoprecipitation with antibodies raised against Tic110, 35S-labelled pSSU was co-immunoprecipitated (Figure 1E), while in the absence of a cross-linker, pSSU was rarely detectable (Figure 1E). Immunodetection with antiserum against Tic110 verified that both assays resulted in comparable amounts of immunoprecipitated Tic110 (data not shown).
Fig. 1. Binding of preprotein to isolated inner envelope vesicles. (A) Inner envelope vesicles (5 µg protein), the 35S-labelled small subunit of the ribulose bisphosphate carboxylase (SSU) and the light harvesting complex protein II (LHCP) were incubated. Only the preproteins (pSSU, pLHCP), not the mature forms (mSSU, mLHCP), were re-isolated together with inner envelope vesicles, i.e. 20% (SSU) or 10% (LHCP) of the translation product used for binding is shown in the left lane (–). An X-ray film is shown. (B) Purity of isolated inner envelope vesicles. Outer envelope vesicles (oe), inner envelope vesicles (ie) and thylakoids (thy) equivalent to 0.5 µg protein, respectively, were subjected to immunodecoration. Antisera raised against marker proteins of the outer envelope (Toc86, Toc75, Toc34 and OEP24), the inner envelope (Tic110) and the thylakoids (LHCP) were used. (C) Binding of preproteins is dependent on proteins at the inner envelope. Treatment of inner envelope vesicles with trypsin (Try) prior to binding decreased the binding of pSSU, pLHCP, the preprotein of ferredoxin (pFd) and the 23 kDa protein of the oxygen-evolving complex (pOE23). The left lane comprises 20% translation product. An X-ray film is shown. (D) Tic110 and Tic40 are protease-accessible at inner envelope vesicles. A ratio of 25 ng trypsin/µg inner envelope protein was used. The samples were subjected to SDS–PAGE, transferred to nitrocellulose and entire lanes were incubated with antisera raised against (α) Tic110, Tic22, Tic55, and Tic40. The inner envelope vesicles (8%) used for the binding assay with (+) or without (–) trypsin treatment were analysed. (E) Binding of pSSU to Tic110. Inner envelope vesicles were incubated with radiolabelled pSSU prior to cross-linking with sodium tetrathionate. After solubilization with SDS, the proteins were immunoprecipitated with antiserum raised against Tic110. The cross-linked product was cleaved with β-mercaptoethanol. An aliquot of each fraction (T, FT, W, E) was subjected to SDS–PAGE and analysed by autoradiography.
Binding of a preprotein to Tic110 proteoliposomes
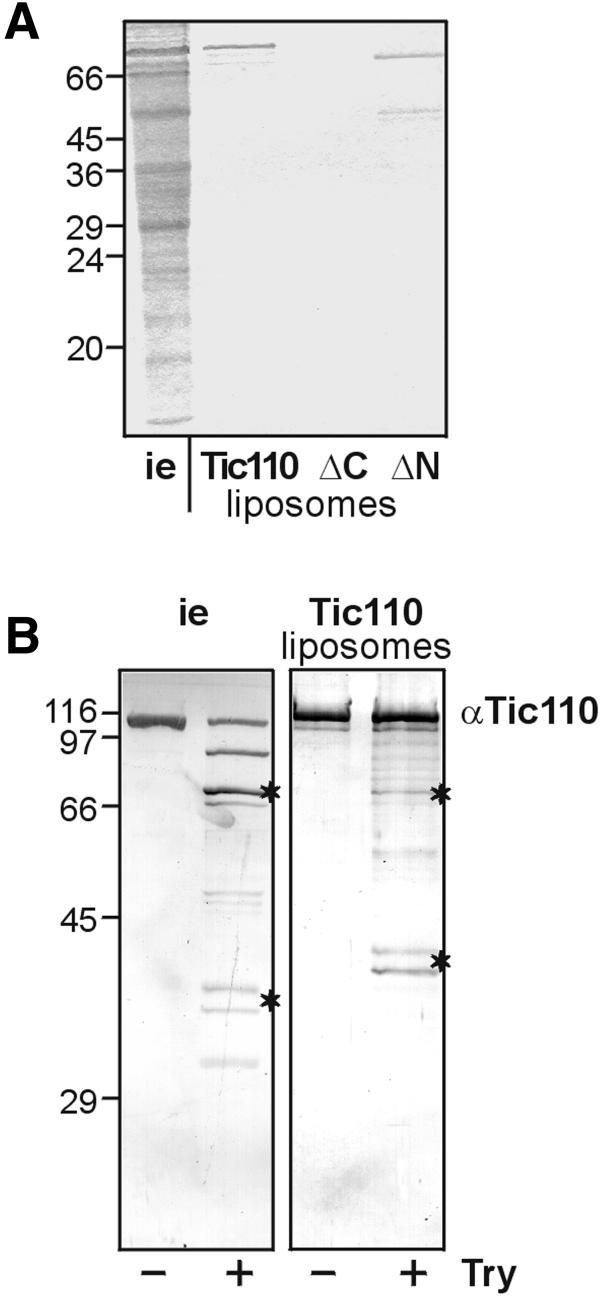
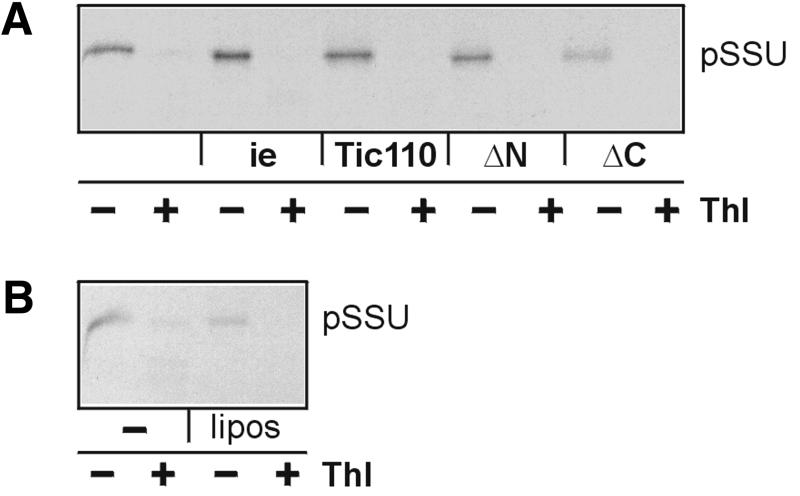
To study the putative role of Tic110 in preprotein binding in more detail, it was expressed in E.coli cells with an N-terminal poly(His) tag and purified by a two-step procedure (see Materials and methods). The purified, urea-denatured protein was mixed with the non-ionic detergent nonanoyl-N-methylglucamide (Mega-9) and azolectine from soybean. The detergent was removed by dialysis, and the purity of the protein reconstituted into liposomes was determined by SDS–PAGE (Figure 2A). Due to its poly(His) tag recombinant Tic110 migrated slightly higher than endogenous Tic110. The same purification procedure except the size exclusion chromatography was used to purify and to reconstitute deletion mutants, which lacked either the C-terminal amino acids Glu232–Phe958 (ΔC) or the N-terminal amino acids Ser1–Phe177 (ΔN) (Figure 2A). The ΔC mutant protein could not be reconstituted, though the affinity chromatography yielded a high amount of protein (data not shown). Proteoliposomes of the ΔN mutant protein contained two bands: the upper one migrated at its expected position, and the less abundant one co-migrated at ∼50 kDa. The latter represents a shorter form of ΔN-Tic110, as demonstrated by immunoblotting using an antiserum against Tic110 (data not shown). To assess the quality of reconstitution, the accessibility of Tic110 in proteoliposomes or inner envelope vesicles towards treatment with the protease trypsin was compared. Though the proteolytic pattern obtained after immunodecoration did not match exactly, the similarity is high (Figure 2B). Characteristic features of the pattern from both endogenous Tic110 and proteoliposomes were products of ∼70 kDa and a doublet of peptide fragments that migrated <45 kDa keeping a constant distance. These bands represented products that were highly resistant against protease treatment indicating that Tic110 took a similar folding in inner envelope vesicles and proteoliposomes. pSSU interacted with proteoliposomes of Tic110 and the ΔN mutant protein (Figure 3A). After binding of pSSU the proteoliposomes were mixed with a 10-fold excess of protein-free liposomes that were resuspended in a buffer of lower density. The protein-free low density liposomes compete for pSSU that only associates with the lipids of the membrane surface. Finally, the proteoliposomes were recovered by centrifugation and the binding of pSSU was assessed after SDS–PAGE. pSSU maintained a loosely folded state during binding as shown by protease treatment (Figure 3A). Proteoliposomes produced with the ΔC mutant did not contain a visible amount of reconstituted protein (Figure 2A). Therefore, binding of 35S-labelled pSSU was hardly detected (Figure 3A). This result was similar to protein-free liposomes that bind only a minor amount of 35S-labelled pSSU (Figure 3B). Furthermore, mSSU interacted neither with proteoliposomes nor with protein-free liposomes (data not shown). We conclude that recombinant Tic110 binds 35S-labelled pSSU in a similar way as it binds to isolated inner envelope vesicles.
Fig. 2. Reconstitution of Tic110 into liposomes. (A) Tic110 and the mutant proteins (ΔN, ΔC) containing an N- or C-terminal poly(His) tag, respectively, were reconstituted into liposomes. The purity of the proteins and the efficiency of reconstitution was finally examined by 25% High-Tris–Urea PAGE. As a control, inner envelope vesicles (i.e. 10 µg protein) were subjected to electrophoresis. A Coomassie Blue-stained gel is shown. The masses of the molecular weight standards are given in kDa at the left side. (B) Protease treatment of inner envelope vesicles and Tic110 liposomes. Inner envelope vesicles (i.e. 2.5 µg protein) and Tic110 liposomes (25 ng protein) were treated with 25 ng trypsin (Try)/µg protein. After SDS–PAGE (10% acrylamide), the protein was transferred to nitrocellulose. The proteolytic pattern was detected with antiserum against (α) Tic110. Characteristic bands at 70 kDa and a doublet at ∼45 kDa are indicated by asterisks. The masses of the molecular weight standards are given in kDa at the left side.
Fig. 3. Binding of preprotein to Tic110 and mutant proteins reconstituted into liposomes. (A) After binding, 35S-labelled pSSU was re-isolated together with inner envelope vesicles (5 µg protein), Tic110-proteoliposomes and ΔN-proteoliposomes (50 ng protein), but was not recovered together with ΔC-proteoliposomes. After binding and re-isolation, half of each sample was treated with thermolysin (Thl). The translation product (20%) used for binding is shown in the left lane (–). (B) As a control, the same experiment was performed with liposomes that did not contain protein, but were treated like proteoliposomes. The translation product (20%) used for binding is shown in the left lane (–). Binding was examined by 25% High-Tris–Urea PAGE. X-ray films are shown.
Recombinant Tic110 forms a transit peptide-sensitive channel
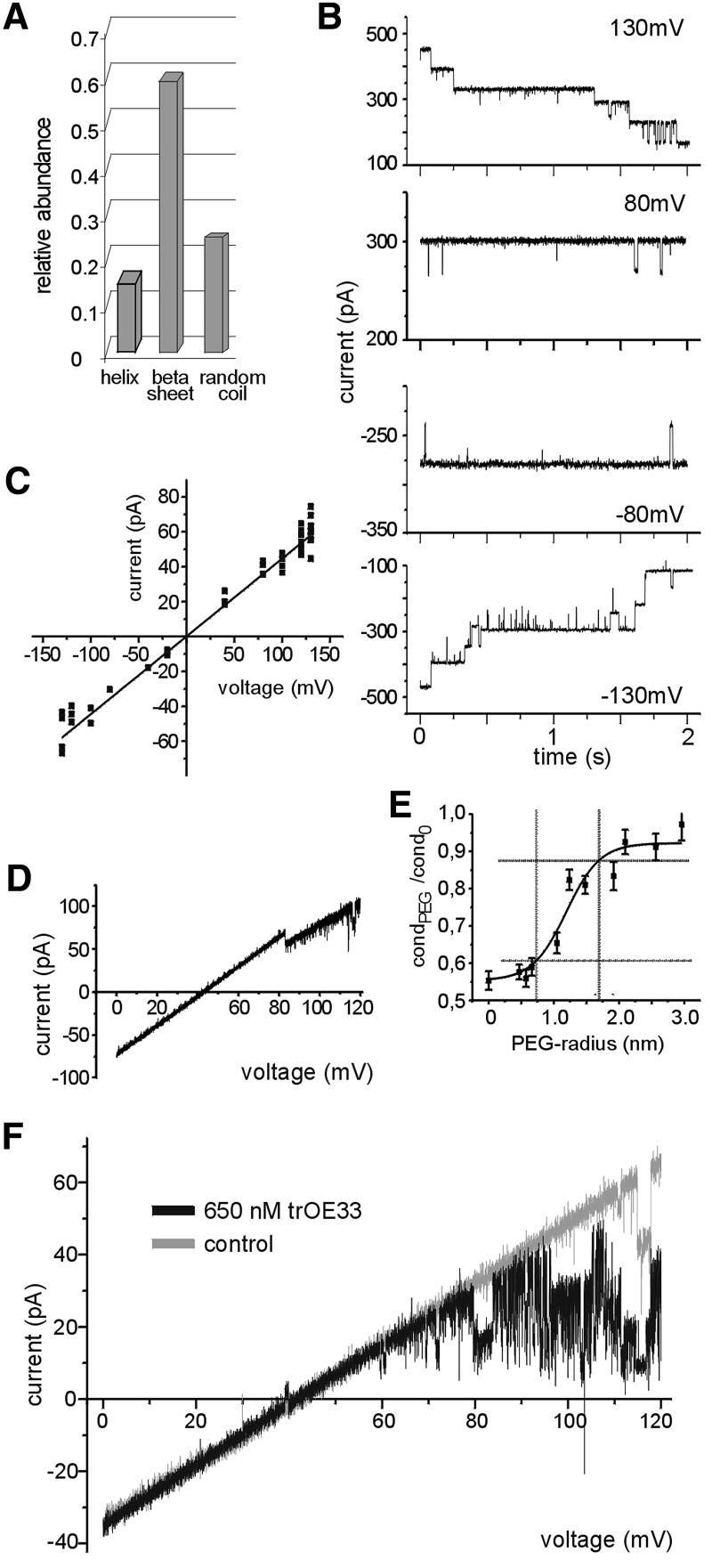
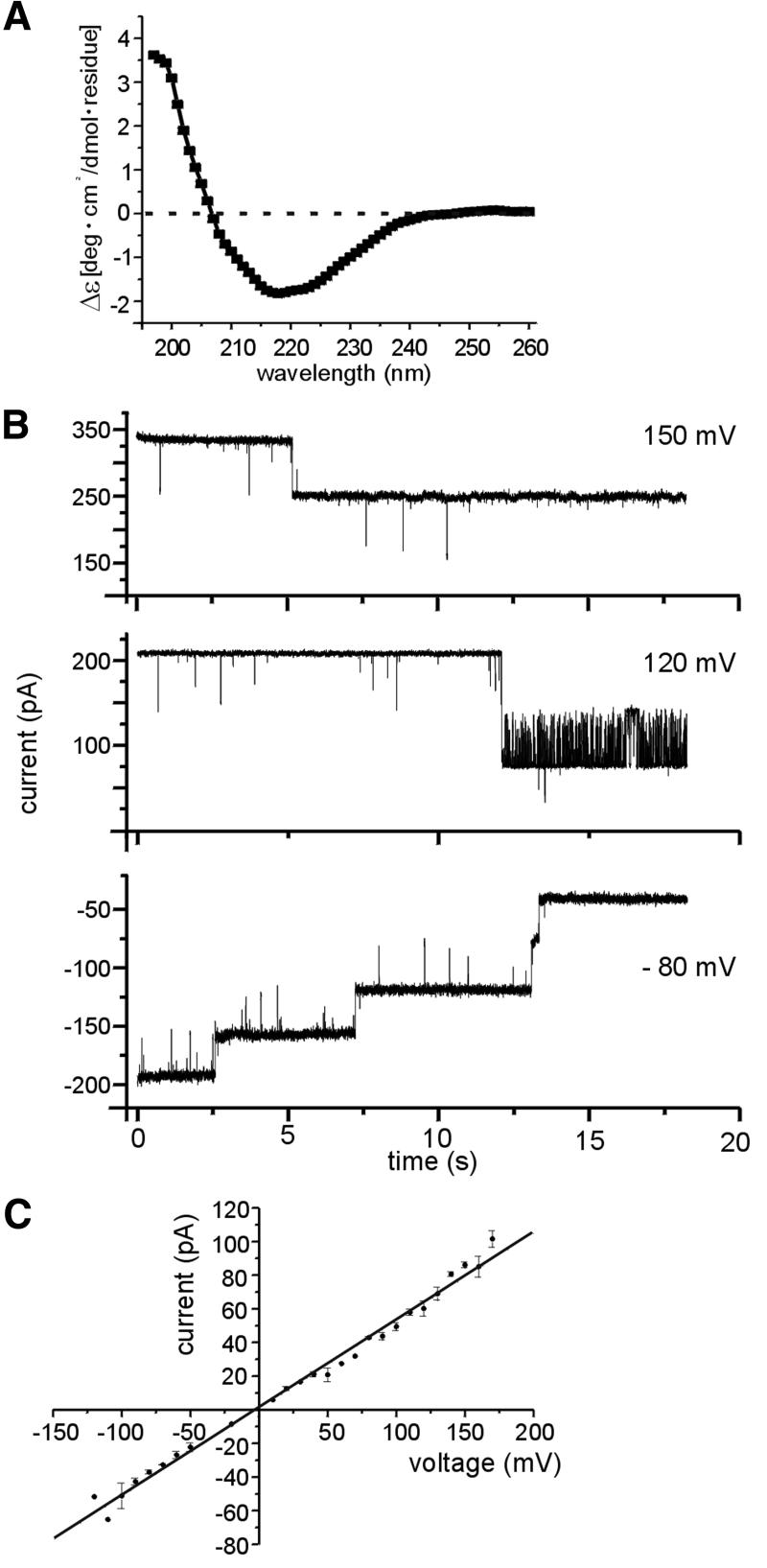
Computer analysis of the amino acid sequence led to the assumption that one or two hydrophobic transmembrane domains close to the N-terminus take an α-helical structure, which anchors Tic110 in the inner envelope membrane (Lübeck et al., 1996; Kessler and Blobel, 1996). The C-terminus was believed to form a large globular domain at the membrane surface. This assumption was supported by the observation that minute amounts of protease were sufficient to obtain multiple proteolytic products of Tic110 (Lübeck et al., 1996; Figure 2B). Interestingly, these proteolytic products withstood higher protease concentrations up to 1 µg trypsin/mg inner envelope protein (Lübeck et al., 1996; Figure 2B), indicating that in addition to the hydrophobic N-terminal domain, stretches at the C-terminus might be deeply embedded in the membrane. Therefore, a circular dichroism spectrum of recombinant Tic110 after dilution in Mega-9 was recorded to obtain insight into the overall secondary structure. Based on the spectrum, the abundance of secondary structures was calculated using a neural network technique, which includes the circular dichroism spectra of known protein structures (Dalmas et al., 1994). Only a small amount of α-helical structure (≨15%) was observed, while the predominant structure was β-sheets (≥60%) (Figure 4A). In accordance with the results obtained from the circular dichroism spectra, a pattern typical for β-strand, membrane-localized proteins was observed predicting the secondary structure of Tic110 by a neural network that comprises bacterial outer membrane-localized β-strand proteins with known structure (Diederichs et al., 1998). Since other protein translocation pores, such as the translocon at the chloroplast outer envelope membrane Toc75 and the translocase of the outer mitochondrial membrane Tom40, are mainly formed by β-sheets (Hinnah et al., 1997; Hill et al., 1998), it was tempting to investigate whether recombinant Tic110 could form a channel. After fusion of Tic110-containing liposomes with a planar bilayer ion-channel activity was observed (Figure 4B). At a low membrane potential (50≥ Vh≥ –50 mV), the channels remained open, while at higher potentials, their open probability decreased. In general, the gating activity of the channel was low and subconductance levels were rarely observed (Figure 4B). With 250 mM KCl buffer on both sides of the membrane, a single fully open channel revealed a linear current–voltage relationship with a slope conductance of Λ = 446 ± 9 pS (Figure 4C). The high conductance of the channel formed by Tic110 was comparable with the values obtained for Toc75 and Tom40 (Bölter et al., 1998; Hill et al., 1998). With asymmetric buffer concentration (250 mM KCl cis/20 mM KCl trans), the Tic110 channel showed strong cation selectivity, as indicated by the reversal potential of Erev = 44 ± 1.6 mV (Figure 4D). As a function of Erev, the Goldman-Hodgkin-Katz equation (Hille, 1992) yielded a permeability ratio of PK+:PCl– ∼10:1. In order to assess the size of the channel formed by recombinant Tic110, we used the polymer size exclusion method (Krasilnikov et al., 1992). The size of the narrowest and the widest opening was obtained by the distribution of differently sized non-electrolyte polyethyleneglycol (PEG) molecules in the pore (Krasilnikov et al., 1992; Smart et al., 1997). At the lower cut-off, which corresponds to the narrowest opening of the channel, small PEG molecules were gradually excluded from the pore, reducing the current passing through the channel. With larger PEG molecules, which are completely excluded from the pore, the conductance of the channel was no longer affected (Figure 4E). The Tic110 channel has at least a diameter of 15 Å and at most 31 Å, indicating that the channel does not have a uniform cylinder-like shape. However, the narrowest opening of 15 Å would be sufficient to allow the translocation of a partially folded protein. We further tested the sensitivity of the Tic110 channel to the positive-charged transit peptide of the 33 kDa protein of the oxygen-evolving complex (trOE33). Added at the trans side of the membrane (20 mM KCl trans/250 mM KCl cis), purified trOE33 induced a voltage-dependent flickering and blocked the Tic110 channel (Figure 4F). Already at lower concentration, with 130 nM trOE33, a significant effect was achieved (data not shown), but with 650 nm trOE33, the Tic110 channel was already blocked at a lower membrane potential, indicating a saturated interaction of the transit peptide with the channel. Blocking of the Tic110 channel was accompanied by a lower open probability and a significantly stronger gating activity of the channel. The flickering indicates that the transit peptide binds to the channel and thereby induces fast openings and closings. In the absence of the transit peptide, a flickering of the Tic110 channel was not observed (Figure 4F). Accordingly, we conclude that Tic110 specifically interacted with the transit peptide. With control peptides lacking the transit peptide, neither a block of the channel nor changes in the gating activity of the channel were observed, even at a concentration 100-fold higher than the transit peptide (data not shown).
Fig. 4. Tic110 forms a cation-selective channel sensitive to a chloroplast transit peptide. (A) Based on a circular dichroism spectra of Tic110 solved in 1% Mega-9, 50 mM KiPO4 pH 7.2, the relative abundance of secondary structures was calculated using a neural network (Dalmas et al., 1994). (B) Current traces from a bilayer containing multiple copies of the channel formed by recombinant Tic110, at different membrane potentials with 250 mM KCl, 2 mM CaCl2 and 10 mM MOPS–Tris pH 7.2 at both sides of the membrane. Subconductance levels were rarely observed. The value of the membrane potential applied is indicated at the right side. (C) Current–voltage relationship of a fully open single channel. The trans and the cis chamber symmetrically contain 250 mM KCl. The values were determined on average of four independent bilayers. The slope conductance of Λ = 446 ± 9 pS indicates a high conductance channel. (D) Ion selectivity of the Tic110 channel. In response to a voltage gradient starting at zero to a positive holding potential, the permeation of cations was preferred over anions. With asymmetric buffers at both sides of the bilayer (250 mM KCl cis/20 mM KCl trans) the channel had a reversal potential of Erev = 44 ± 1.6 mV (on average of 12 independent bilayers). (E) The inner diameter of the Tic110 channel. The conductance of the Tic110 channel in the presence of differently sized non-electrolyte PEG molecules was measured. The values of the narrowest part (filter) and the widest part (vestibule) of the pore were estimated. The hydronamic radius of the PEG molecules versus the quotient of the conductance in the presence of the non-electrolyte PEG (condPEG) and the conductance without PEG (cond0) is shown. Dashed lines indicate the narrowest and the widest opening. (F) The Tic110 channel is sensitive to chloroplast transit peptide. Purified transit peptide (trOE33) was added at the trans side (250 mM KCl) of the bilayer containing Tic110 and stirred for 10 s. Next, a voltage sweep (Δ = 10 mV/s) was applied and a block of the Tic110 channel starting at a membrane potential of ∼70 mV was observed (black). In the absence of trOE33, a linear current–voltage relationship occurred (gray). The activity of a bilayer containing five channels is shown.
Next, the structural and electrophysiological properties of the ΔN mutant Tic110, which after reconstitution into liposomes interacts with pSSU, were examined following the line as described above. Like the wild-type Tic110, the ΔN mutant protein solved in N-decyl-β-d-maltopyranoside has mainly a β-sheet conformation as indicated by a circular dichroism spectrum (Figure 5A). The ion channel activity of the ΔN mutant resembled the properties of the wild-type Tic110 channel. After fusion of ΔN mutant Tic110-containing liposomes with a planar bilayer, an ion channel was formed, which was mainly open at a lower membrane potential. The gating activity of the channel was low and subconductance levels were rarely observed (Figure 5B). A linear current–voltage relationship with a slope conductance of Λ = 520 ± 10 pS (Figure 5C) was established after recording a single fully open ΔN mutant Tic110 channel in the presence of 250 mM KCl at both sides of the membrane.
Fig. 5. Characterization of a Tic110 ΔN mutant. (A) Circular dichroism spectrum of the ΔN mutant Tic110 solved in 2% N-decyl-β-d-maltopyranoside and 50 mM KiPO4 pH 7.2. (B) Current traces from a bilayer containing multiple copies of the channel formed by the ΔN mutant Tic110. At both sides of the membrane, 250 mM KCl, 2 mM CaCl2 and 10 mM MOPS–Tris pH 7.2 were present. With different positive and negative membrane potentials, subconductance levels of the ΔN mutant channel were rarely observed. (C) Current–voltage relationship of a fully open single ΔN mutant channel. The trans and the cis chamber symmetrically contain 250 mM KCl. The values were determined on average of five independent bilayers. The slope conductance of 520 ± 10 pS indicates a high conductance channel.
In summary, recombinant Tic110 forms a voltage-dependent cation-selective channel in vitro, which specifically responds to a transit peptide, supporting the idea that Tic110 builds up the protein translocation pore at the inner envelope membrane.
Isolated inner envelope membranes contain a cation-selective channel sensitive to a transit peptide
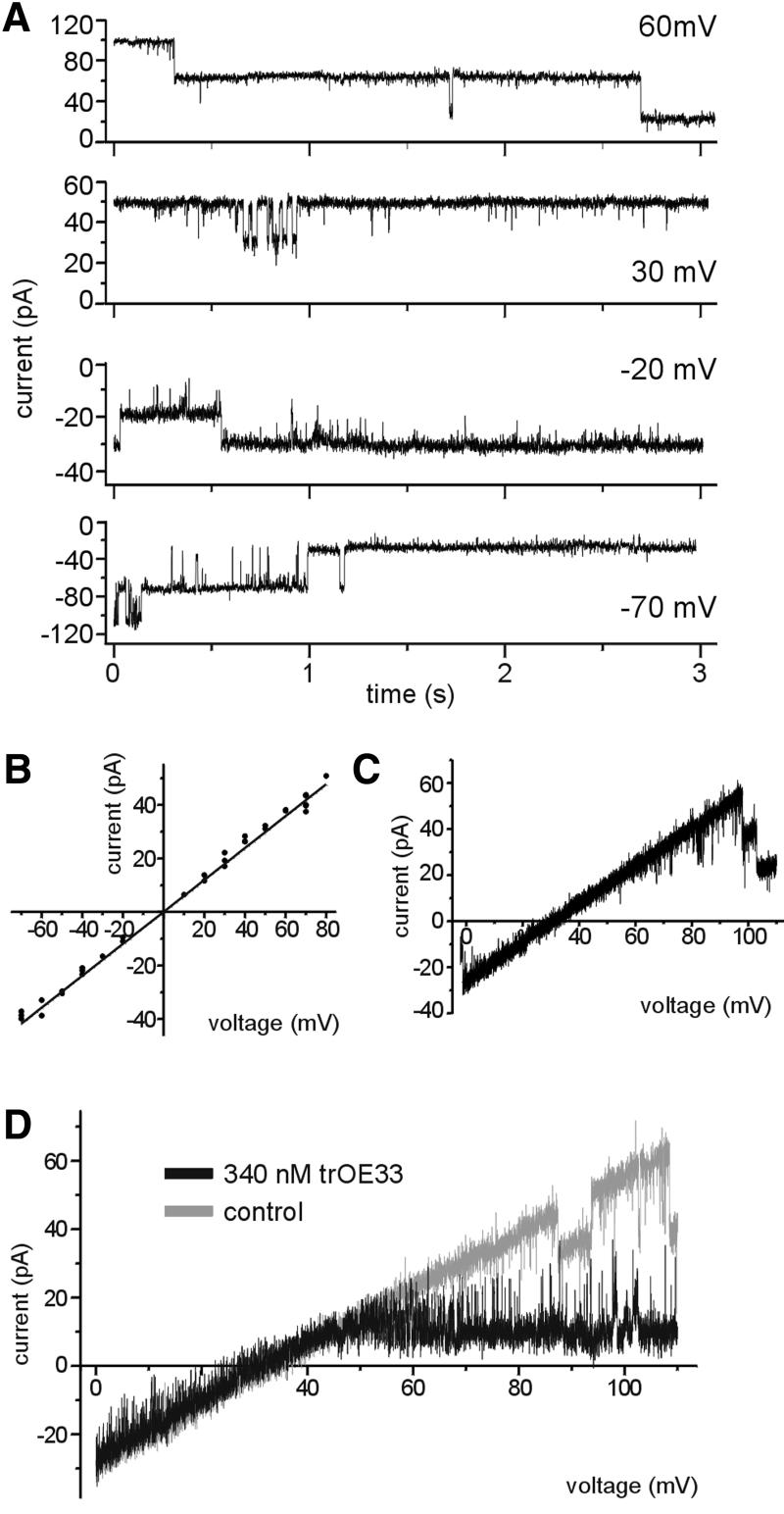
In order to verify the results obtained with the recombinant protein, the channel properties of wild-type Tic110 from inner envelope vesicles were investigated. Tic110 was isolated from its natural source with high purity (data not shown), however, the reconstitution of an active Tic110 channel into the lipid bilayer failed. Therefore, purified inner envelope vesicles fused with liposomes were used to investigate the activity of a channel comparable with the channel formed by recombinant Tic110. After fusion of these proteoliposomes to a lipid bilayer, different anion- and cation-selective channels were observed (Mehrle, 2001). The cation-selective channels were classified according to specific characteristics: (i) a low conductance channel highly selective for cations probably represented a potassium channel already described by Heiber et al. (1995); (ii) a high conductance channel (Λ = 680 ± 13 pS) was slightly cation-selective (PK+:PCl– ∼3.5:1), but was not sensitive to transit peptide (Mehrle, 2001); and (iii) a high-conductance cation-selective channel highly sensitive to trOE33 has properties that closely resemble the properties of the channel formed by recombinant Tic110 (see below). With 250 mM KCl on both sides of the membrane, the peptide-sensitive channel at the inner envelope membrane showed a notably low gating frequency (Figure 6A), similar to the channel formed by recombinant Tic110. At a higher positive or negative holding potential, closing of the channel at the inner envelope membrane occurred more often (Figure 6A). In symmetric buffers, a linear current–voltage relationship was observed with a slope conductance of Λ = 600 ± 8 pS (Figure 6B). As deduced from the reversal potential Erev = 34 ± 1.6 mV (Figure 6C), with asymmetric buffers (250 mM KCl cis/20 mM KCl trans) the permeation rate of cations through the channel is five times higher than the rate of anions (PK+:PCl– ∼5.3:1). Furthermore, the addition of 340 nM trOE33 at the trans side of the membrane (20 mM KCl trans/250 mM KCl cis) induced a voltage-dependent block of the channel at the inner envelope membrane (Figure 6D) as already shown for recombinant Tic110 (Figure 4F). Together, these findings provide strong evidence that both the channel formed by recombinant Tic110 and the cation-selective channel described in the inner envelope membrane are identical. In view of the fact that at inner envelope membranes the activity of a channel is probably influenced by associated Tic proteins, small differences from the recombinant Tic110 channel can be expected. In principle, the upper values pointed to an identical function of recombinant Tic110 and the cation-selective channel characterized at inner envelope membranes. The conclusion we draw from these results is that Tic110 functions as preprotein translocation pore at the inner envelope membrane.
Fig. 6. Inner envelope vesicles contain a channel with properties identical to recombinant Tic110. (A) Current traces from a bilayer containing a single Tic110-like channel at different membrane potentials with 250 mM KCl, 2 mM CaCl2 and 10 mM MOPS–Tris pH 7.2 at both sides of the membrane. The value of the membrane potential is indicated at the right side. (B) Current–voltage relationship of a fully open single channel. The trans and the cis chamber symmetrically contain 250 mM KCl. The values are shown on average of three independent bilayers. The slope conductance of Λ = 600 ± 8 pS indicates a high conductance channel. (C) The channel exhibits cation selectivity. Current recordings obtained from a bilayer containing multiple copies of active Tic110 channels in response to a voltage ramp are shown. With asymmetric buffers (250 mM KCl cis/20 mM KCl trans), the reversal potential of 34 ± 1.6 mV was deduced, indicating that the channel is cation-selective. (D) The cation-selective Tic110-like channel at the inner envelope membrane is sensitive to chloroplast transit peptide. With 250 mM KCl cis and 20 mM KCl trans, a voltage sweep (Δ = 10 mV/s) was applied to the membrane and a linear correlation was observed (gray). After addition of transit peptide (trOE33) at the cis side, at a membrane potential of ∼45 mV, the current passing through the channel significantly decreased (black). The activity of a bilayer containing three channels is shown.
Discussion
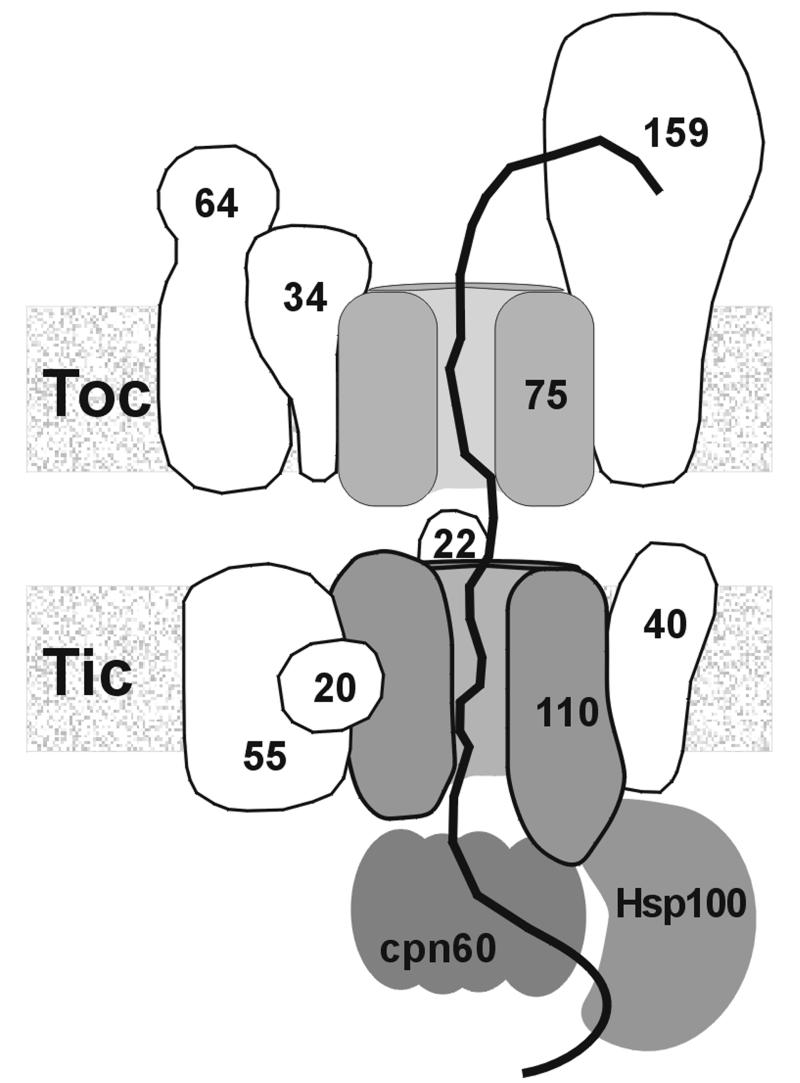
To gain insight into the molecular mechanism of preprotein translocation, we investigated the interaction of chloroplast preproteins with either isolated inner envelope vesicles or recombinant Tic110 reconstituted into liposomes. We found that inner envelope vesicles bound chloroplast preproteins more specifically than the mature forms, indicating that the interaction depends on the transit peptide. However, translocation as judged from the formation of a protease-resistant product of the preprotein was not observed. Likewise, isolated outer membranes of mitochondria only bound, but did not translocate, preproteins destined for the matrix (Mayer et al., 1993). Probably, components essential for translocation are missing because proteins, that in organello are localized in the intermembrane space or the stroma, were removed during preparation of inner envelope vesicles. At isolated outer envelope vesicles of chloroplasts, a complete passage of pSSU to the inside of the vesicles was also not observed (Waegemann and Soll, 1991). However, translocation intermediates of pSSU resistant to protease treatment occurred (Waegemann and Soll, 1991). This finding might be explained by a different set of proteins interacting with pSSU at the outer and inner envelope membranes, respectively, indicating that the molecular mechanism of translocation at the envelope membranes might vary. Protease treatment of the inner envelope vesicles prior to the addition of different preproteins demonstrated that the interaction of both was mainly dependent on proteins on the vesicle’s surface. Probably, the existence of intact Tic110 was required for efficient binding of the preprotein. However, the involvement of Tic40 or another unknown component in binding of the preprotein can not be excluded by this experiment. Preprotein binding is dependent on the presence of Tic110 or the ΔN-Tic110 mutant (Glu232–Phe958). The mSSU did not bind to either proteoliposomes or protein-free liposomes, indicating that Tic110, which has a high abundance of negative-charged amino acids, might interact with the positive-charged transit peptides. Furthermore, we demonstrated that the C-terminal domain of Tic110 maintained the capability to assemble into a lipid bilayer and to bind a preprotein. At both inner envelope vesicles and proteoliposomes, bands of ∼70 and 40 kDa remained highly resistant to protease, supporting the view that both the hydrophobic N-terminal region and several peptide stretches at the C-terminus anchor Tic110 in the membrane. Earlier, Tic110 was proposed to be involved in the formation of contact sites between the envelope membranes because a globular C-terminal domain of Tic110 was suggested to face the intermembrane space (Lübeck et al., 1996). Assuming that the C-terminus of Tic110 protrudes into the stroma (Jackson et al., 1998), a function in recruiting stromal chaperones such as cpn60 and Hsp100 was also suggested (Kessler and Blobel, 1996; Nielsen et al., 1997). However, the capability of isolated Tic110 to bind a preprotein demonstrates that Tic110 has a distinct preprotein binding site besides its role as a translocation pore. Likewise, interaction with a preprotein occurred when the chloroplast protein translocation pore Toc75 (Hinnah et al., 1997) or the mitochondrial protein translocation pore Tom40 (Hill et al., 1998) were reconstituted into liposomes. All functions mentioned above are conceivable assuming that the C-terminal region of Tic110 is not exclusively orientated to one side of the membrane, but spans it several times (Figure 7). This notion is supported by circular dichroism spectra of Tic110 and ΔN mutant Tic110, indicating that the β-sheet conformation is the most abundundant structure of both proteins. Several pore-forming proteins, such as several bacterial porins (Bainbridge et al., 1998) and a voltage-dependent anion channel of mitochondria (Dolder et al., 1999), show a β-barrel conformation consisting of β-sheets. Like Tic110, these proteins have a low overall hydrophobicity. After fusion to a lipid bilayer, Tic110 and ΔN mutant Tic110 constituted a hydrophilic transmembrane pore. A diameter of 15 Å (filter) to 34 Å (vestibule) was calculated for the pore formed by Tic110. A pore width of 20 Å as similarly calculated for the pore of the mitochondrial preprotein translocon at the outer membrane Tom40 (Hill et al., 1998), the mitochondrial preprotein translocon at the inner membrane, the Tim17/23 complex (Lohret et al., 1997) and chloroplast Toc75 (Hinnah et al., 1997) were already sufficient to conduct partially folded preproteins. The Tic110 channel was cation-selective as judged from the permeability ratio. Values similar to Tom40 (Hill et al., 1998) and Toc75 (Hinnah et al., 1997) were obtained. Likewise, Tic110 formed a high-conductance channel (Λ = 446 ± 9 pS). In contrast to Tom40 and Toc75, current traces of Tic110 did not display different subconductance levels and gating events occurred less frequently, indicating that the molecular mechanism behind opening and closure of the Tic110 pore might be different in vivo. After fusion of isolated inner envelope vesicles to a planar lipid bilayer, a channel was identified that had a similar conductance (Λ = 600 ± 8 pS), cation selectivity and voltage-dependence as the channel formed by purified Tic110, supporting the suggestion that both channels are identical. At isolated membrane fractions, the electrophysiological activity of preprotein-conducting pores was also established for the Tim17/23 complex (Lohret et al., 1997), Tom40 (Hill et al., 1998) and Toc75 (Hinnah et al., 1997). The results obtained after reconstitution of recombinant protein reliably correspond to the results obtained after fusion of membrane vesicles in the cases of Tom40 and Toc75. All of the preprotein-conducting channels mentioned above commonly were specifically blocked by a preprotein or transit peptides (Hinnah et al., 1997; Lohret et al., 1997; Hill et al., 1998). The Tic110 channel formed by recombinant protein or found in inner envelope vesicles also displayed a specific block after the addition of purified transit peptide at the trans side of the lipid bilayer. The interaction of the transit peptide with the Tic110 channel transiently occurred in a voltage-dependent manner accompanied by an increased flickering rate. These results indicated that either the transit peptide was not tightly bound or destabilized the open state of the channel. Patch-clamp analysis of the chloroplast outer and inner envelope membranes having a sandwich-like structure, revealed the existence of a low-conductance, anion-selective channel (PIRAC) that reacted to chloroplast transit peptides and was blocked by antiserum against Tic110 (van den Wijngaard and Vredenberg, 1997, 1999; van den Wijngaard et al., 2000). The channel was assigned to the inner envelope membrane on the assumption that the chloroplast outer envelope membrane was permeable to low molecular weight solutes ≥600 Da (Flügge and Benz, 1984). Recently, several highly regulated channels in the outer envelope membrane were described (for a review see Bölter and Soll, 2001), casting some doubt over previous interpretations of the localization of PIRAC. Furthermore, all preprotein translocation channels of chloroplasts and mitochondria characterized so far show cation selectivity (Hinnah et al., 1997; Lohret et al., 1997; Hill et al., 1998), in accordance with the translocation of a positive-charged transit peptide. In contrast, PIRAC exhibited strong anion selectivity (van Wijngaard et al., 2000). Therefore, a direct participation of PIRAC in the translocation of preproteins seems unlikely. The interaction of Tic110 with a preprotein and the electrophysiological properties of the pore formed by Tic110 provide striking evidence that Tic110 builds up at least a major part of the preprotein translocation pore at the chloroplast inner envelope membrane.
Fig. 7. Model of the Tic complex. Tic110 forms at least a major part of the preprotein translocation pore. At the same time Tic110 might be involved in the formation of joint translocation sites together with the Toc complex and in recruiting of stromal chaperones (cpn60, Hsp100). The numbers indicate the calculated molecular weight of the Toc and Tic components.
Materials and methods
Isolation of inner envelope membranes
In principle, intact chloroplasts from 12 to 14-day-old pea plants (Pisum sativum L., var. Golf) were isolated as described previously (Keegstra and Yousif, 1986; Waegemann and Soll, 1991), but the following changes were introduced. All media contained 1.5 mM β-mercaptoethanol, and the sucrose density gradient was layered as follows: 8 ml 0.996 M; 9 ml 0.8 M; 8 ml 0.465 M sucrose.
Binding of preprotein
Binding assays contained 10 µg inner envelope protein or proteoliposomes equivalent to 100 ng protein in 50 µl binding buffer (20 mM MOPS–Tris pH 7.4, 1.25 mM methionine, 1.25 mM cysteine, 0.1% BSA, 1 mM ATP, 1 mM MgCl2) and 1–10% of 35S- or 3H-labelled preprotein translated in reticulocyte lysate in vitro. Before use, the translation product was centrifuged at 250 000 g for 15 min at 4°C. Binding of the supernatant was allowed at 25°C for 7 min. Then, inner envelope vesicles were re-isolated through a 0.5 M sucrose cushion at 100 000 g for 10 min at 4°C. The pellet was resuspended in 0.1 mM CaCl2, 20 mM Tris–HCl pH 8.0. Half of the sample was subjected to protease treatment with thermolysin (15 min, 4°C) while the other half was kept on ice. Binding of preprotein to proteoliposomes was performed as described above, except the binding buffer contained 170 mM sucrose. After binding of the preprotein, proteoliposomes were mixed with a 10-fold amount of liposomes, which were in 100 mM NaCl, 20 mM MOPS–Tris pH 7.4. The sucrose-containing proteoliposomes were recovered by centrifugation at 50 000 g for 30 min at 4°C. The pellet was treated as described before. The binding efficiency was analysed by SDS–PAGE and autoradiography. For protease treatment prior to binding of preprotein, inner envelope vesicles were resuspended in 50 µl buffer (0.1 mM CaCl2, 20 mM Tris–HCl pH 8.0). Trypsin was added to a concentration of 25 ng/µg inner envelope protein and the reaction was allowed at 25°C for exactly 90 s. Then, a 5-fold molar excess of soybean trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride terminated the digestion. Finally, inner envelope vesicles were recovered after centrifugation through a 0.5 M sucrose cushion. The pellet was resuspended in binding buffer. A portion was subjected to SDS–PAGE, transferred to nitrocellulose, and antisera against Tic110, Tic55, Tic40 and Tic22 were used to examine the effect of the protease on the inner envelope proteins.
The binding assay for cross-linking and co-immunoprecipitation contained 20 µg inner envelope protein in 25 mM HEPES–NaOH pH 7.6. Inner envelope vesicles and [35S]pSSU were incubated for 5 min at 25°C. After addition of 0.1% SDS, the reaction was incubated for another 5 min at 25°C. The cross-linking reaction was then initiated by addition of 1 mM sodium tetrathionate and carried out for 30 min at 20°C. Cross-linking was terminated by solubilization with 2% SDS (final concentration). For co-immunoprecipitation, three independent samples were pooled, diluted 1:10 with IP buffer (25 mM HEPES–NaOH, 150 mM NaCl, 0.1% egg albumin pH 7.6) and incubated with 5 µl of antiserum against Tic110 for 90 min at 20°C, followed by an 1 h incubation with pre-washed protein A–Sepharose CL-4B (Pharmacia, Inc.). The Sepharose was then washed five times with IP buffer and the elution was performed using SDS–PAGE sample buffer containing 10 mM β-mercaptoethanol to cleave the cross-link. One per cent of the total cross-linking reaction, the flow-through, the final washing step and the entire eluate were subjected to SDS–PAGE and autoradiography.
Expression of Tic110 and its mutants
The cDNA fragment encoding the amino acids Ser1–Phe958 was subcloned into the vector pET14b (Novagen, Madison, WI). The cDNA fragments, encoding the amino acids Val178–Phe958 (ΔN mutant) and Ser1–Thr231 (ΔC mutant) of the mature Tic110, were subcloned into the vector pET21d (Novagen) (for details, see Lübeck et al., 1996). All constructs were transformed into E.coli BL21 (DE3), respectively, and expressed as inclusion bodies. Inclusion bodies were extensively washed in buffer containing 0.1% (w/w) Nonidet P40 and solubilized in guanidine buffer [6 M guanidine–HCl, 1 M NaCl, 20 mM Tris, 10 mM dithiothreitol (DTT), adjusted to pH 7.2 with 1 M NaH2PO4]. The supernatant was loaded onto a size exclusion column (TosoHaas TSK-GEL G3000SW, Stuttgart, Germany), from which it was eluted with 7 M urea, 1 M NaCl and 20 mM Tris, adjusted to pH 7.2 with 1 M NaH2PO4. Fractions enriched in Tic110 were pooled and loaded onto a Ni-NTA agarose (Qiagen, Hilden, Germany). The protein was eluted with 6 M urea, 1 mM DTT, 100 mM imidazol and 20 mM Tris, adjusted to pH 7.6 with 1 M NaH2PO4. The inclusion bodies, containing mutant proteins, were solubilized in guanidine buffer and further purified by metal-affinity chromatography. Mature Tic110 contained an N-terminal poly(His) tag, the ΔN mutant protein and the ΔC mutant protein a C-terminal poly(His) tag.
Reconstitution of proteins into liposomes
Unilamellar liposomes were obtained from azolectin (Sigma, type IV S) as described (Hinnah et al., 1997). Purified proteins in urea were mixed with liposomes. The final volume was 1.5 ml, and contained 15 mg lipid, 1.5 mg protein, 80 mM Mega-9 and 5 mM β-mercaptoethanol. By the dialysis technique, the reconstitution was achieved (Hinnah et al., 1997). Proteoliposomes that were used for protease treatment or binding assays were dialysed against 170 mM sucrose and 20 mM MOPS–Tris pH 7.4. Free protein was removed by centrifugation at 50 000 g for 30 min at 2°C. Next, the suspension was extruded through a 100 nm mash (LiposoFast, AvestinInc., Ottawa, Canada). The proteoliposomes were mixed with a 10-fold excess of low-density liposomes (100 mM NaCl, 20 mM MOPS–Tris pH 7.4) to remove protein that was only loosely attached to the lipid surface. After centrifugation, the pellet containing the proteoliposomes, was resuspended in 170 mM sucrose, 1 mM DTT and 20 mM MOPS–Tris pH 7.4. The quality of the reconstitution was examined by Coomassie Blue staining after SDS–PAGE and by protease treatment. Proteoliposomes were treated with 25 ng trypsin/µg protein for 10 min at 4°C. The reaction was stopped as described before.
Before electrophysiological measurements, inner envelope vesicles equivalent to 60 µg protein were mixed with 3 mg liposomes and 90 µl buffer (2 mM CaCl2, 5 mM DTT, 10 mM MOPS–Tris pH 7.2). After sonication the proteoliposomes were frozen in liquid nitrogen. A freeze/thaw cycle was repeated twice before use.
Electrophysiological measurements
Planar lipid bilayers were produced using the painting technique (Mueller et al., 1963). The measurement was performed as described previously (Steinkamp et al., 2000).
Miscellaneous
Circular dichroism spectra were recorded using a Jasco-J-600A spectropolarimeter as described previously (Hinnah et al., 1997). The generation of antisera raised against Tic110, Tic55 and Tic40 has been described previously (Lübeck et al., 1996; Caliebe et al., 1997; Stahl et al., 1999). The antiserum against the preprotein of Tic22 was raised using recombinant Arabidopsis thaliana Tic22 (Accession no. AA404873). SDS–PAGE and western blotting were performed according to our published procedures (Lübeck et al., 1996). The gels were dried and exposed either to an X-ray film (BioMax MR, Kodak, UK) or a phosphorimager (FLA-3000 RGB, Fuji). The software AIDA 2.11 was used for quantification.
Acknowledgments
Acknowledgements
The excellent technical assistance of K.Bieberich and the help of M.Bohnsack is gratefully acknowledged. This work was supported in part by the grants of the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
References
- Bainbridge G., Gokce,I. and Lakey,J.H. (1998) Voltage gating is a fundamental feature of porin and toxin β-barrel membrane channels. FEBS Lett., 431, 305–308. [DOI] [PubMed] [Google Scholar]
- Bölter B. and Soll,J. (2001) Ion channels in the outer membranes of chloroplasts and mitochondria: open doors or regulated gates? EMBO J., 20, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölter B., Soll,J., Schulz,A., Hinnah,S. and Wagner,R. (1998) Origin of a chloroplast protein importer. Proc. Natl Acad. Sci. USA, 95, 15831–15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe A., Grimm,R., Kaiser,G., Lübeck,J., Soll,J. and Heins,L. (1997) The chloroplastic protein import machinery contains a Rieske-type iron-sulfur cluster and a mononuclear iron-binding protein. EMBO J., 16, 7342–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmas B., Hunter,G.J. and Bannister,W.H. (1994) Prediction of protein secondary structure from circular dichroism spectra using artificial neural network techniques. Biochem. Mol. Biol. Int., 34, 17–26. [PubMed] [Google Scholar]
- Diederichs K., Freigang,J., Umhau,S., Zeth,K. and Breed,J. (1998) Prediction by a neural network of outer membrane β-strand protein topology. Protein Sci., 7, 2413–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M., Zeth,K., Tittmann,P., Gross,H., Welte,W. and Wallimann,T. (1999) Crystallization of the human, mitochondrial voltage-dependent anion-selective channel in the presence of phospholipids. J. Struct. Biol., 127, 64–71. [DOI] [PubMed] [Google Scholar]
- Flügge U.I. and Benz,R. (1984) Pore-forming activity in the outer membrane of the chloroplast envelope. FEBS Lett., 169, 85–89. [Google Scholar]
- Heiber T., Steinkamp,T., Hinnah,S., Schwarz,M., Flügge,U.I., Weber,A. and Wagner,R. (1995) Ion channels in the chloroplast envelope membrane. Biochemistry, 34, 15906–15917. [DOI] [PubMed] [Google Scholar]
- Hill K., Model,K., Ryan,M.T., Dietmeier,K., Martin,F., Wagner,R. and Pfanner,N. (1998) Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature, 395, 516–521. [DOI] [PubMed] [Google Scholar]
- Hille B. (1992) Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA, pp. 83–115.
- Hinnah S.C., Hill,K., Wagner,R., Schlicher,T. and Soll,J. (1997) Reconstitution of a chloroplast protein import channel. EMBO J., 16, 7351–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.T., Froehlich,J.E. and Keegstra,K. (1998) The hydrophilic domain of Tic110, an inner envelope membrane component of the chloroplastic protein translocation apparatus, faces the stromal compartment. J. Biol. Chem., 273, 16583–16588. [DOI] [PubMed] [Google Scholar]
- Keegstra K. and Yousif,A.E. (1986) Isolation and characterization of chloroplast envelope membranes. Methods Enzymol., 118, 316–325. [Google Scholar]
- Kessler F. and Blobel,G.(1996) Interaction of the protein import and folding machineries in the chloroplast. Proc. Natl Acad. Sci. USA, 93, 7684–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Budd,D., Wu,C., Seibert,F., Kourtz,L. and Ko,Z.W. (1995) Isolation and characterization of a cDNA clone encoding a member of the Com44/Cim44 envelope components of the chloroplast protein import apparatus. J. Biol. Chem., 270, 28601–28608. [DOI] [PubMed] [Google Scholar]
- Kouranov A. and Schnell,D.J. (1997) Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J. Cell Biol., 139, 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranov A., Chen,X., Fuks,B. and Schnell,D.J. (1998) Tic20 and Tic22 are new components of the protein import apparatus at the chloroplast inner envelope membrane. J. Cell Biol., 143, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasilnikov O.V., Sabirov,R.Z., Ternovsky,V.L., Merzliak,P.G. and Muratkhodjaev,J.N. (1992) A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol. Immunol., 5, 93–100. [DOI] [PubMed] [Google Scholar]
- Lohret T.A., Jensen,R.E. and Kinally,K.W. (1997) Tim23, a protein import component of the mitochondrial inner membrane, is required for normal activity of the multiple conductance channel, MCC. J. Cell Biol., 137, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübeck J., Soll,J., Akita,M., Nielsen,E. and Keegstra,K. (1996) Topology of IEP110, a component of the chloroplastic protein import machinery present in the inner envelope membrane. EMBO J., 15, 4230–4238. [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Lill,R. and Neupert,W. (1993) Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J. Cell Biol., 121, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrle A. (2001) Electrophysiological and biochemical characterisation of recombinant and native channel-proteins of the inner envelope membrane of chloroplasts and the cyanobacterium Synechocystis. PhD thesis, University of Osnabrück, Osnabrück, Germany.
- Mueller P., Rudin,O.D., Tien,H. and Wescott,W.C. (1963) Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem., 67, 534–535. [Google Scholar]
- Nielsen E., Akita,M., Davila-Aponte,J. and Keegstra,K. (1997) Stable association of chloroplastic precursors with protein-translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J., 16, 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P., Meathrel,K. and Ko,K. (1997) A component of the chloroplast protein import apparatus functions in bacteria. J. Biol. Chem., 272, 25623–25627. [DOI] [PubMed] [Google Scholar]
- Perry S.E. and Keegstra,K. (1994) Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell, 6, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. and Dobberstein,B. (1996) Common principles of protein translocation across membranes. Science, 271, 1519–1526. [DOI] [PubMed] [Google Scholar]
- Segui-Real B., Kispal,G., Lill,R. and Neupert,W. (1993) Functional independence of the protein translocation machineries in mitochondrial outer and inner membranes: passage of preproteins through the intermembrane space. EMBO J., 12, 2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O.S., Breed,J., Smith,G.R. and Sansom,M.S. (1997) A novel method for structure-based prediction of ion channel conductance properties. Biophys. J., 72, 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl T., Glockmann,C., Soll,J. and Heins,L. (1999) Tic40, a new ‘old’ subunit of the chloroplast protein import. J. Biol. Chem., 274, 37467–37472. [DOI] [PubMed] [Google Scholar]
- Steinkamp T., Hill,K., Hinnah,S.C., Wagner,R., Röhl,T., Pohlmeyer,K. and Soll,J. (2000) Identification of the pore-forming region of the outer chloroplast envelope protein OEP16. J. Biol. Chem., 275, 11758–11764. [DOI] [PubMed] [Google Scholar]
- Sveshnikova N., Soll,J. and Schleiff,E. (2000) Toc34 is a preprotein receptor regulated by GTP and phosphorylation. Proc. Natl Acad. Sci. USA, 97, 4973–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard P.W.J. and Vredenberg,W.J. (1997) A 50-pico siemens anion channel of the chloropast envelope is involved in chloroplast protein import. J. Biol. Chem., 272, 29430–29433. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard P.W.J. and Vredenberg,W.J. (1999) The envelope anion channel involved in chloroplast protein import is associated with Tic110. J. Biol. Chem., 274, 25201–25204. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard P.W.J., Demmers,J.A.A., Thompson,S.J., Wienk, H.L.J., de Kruijff,B. and Vredenberg,W.J. (2000) Further analysis of the involvement of the envelope anion channel PIRAC in chloroplast protein import. Eur. J. Biochem., 267, 3812–3817. [DOI] [PubMed] [Google Scholar]
- Waegemann K. and Soll,J. (1991) Characterization of the protein import apparatus in isolated outer envelopes of chloroplasts. Plant J., 1, 149–158. [Google Scholar]
- Wu C., Seibert,F. and Ko,K. (1994) Identification of chloroplast envelope proteins in close physical proximity to a partially translocated chimeric precursor protein. J. Biol. Chem., 269, 32264–32271. [PubMed] [Google Scholar]